Pendrin, encoded by the Pendred syndrome gene, resides in the apical region of renal intercalated cells and mediates bicarbonate secretion
- PMID: 11274445
- PMCID: PMC31206
- DOI: 10.1073/pnas.071516798
Pendrin, encoded by the Pendred syndrome gene, resides in the apical region of renal intercalated cells and mediates bicarbonate secretion
Abstract
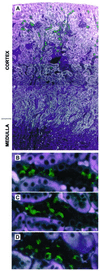
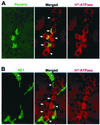
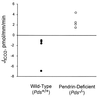
Pendrin is an anion transporter encoded by the PDS/Pds gene. In humans, mutations in PDS cause the genetic disorder Pendred syndrome, which is associated with deafness and goiter. Previous studies have shown that this gene has a relatively restricted pattern of expression, with PDS/Pds mRNA detected only in the thyroid, inner ear, and kidney. The present study examined the distribution and function of pendrin in the mammalian kidney. Immunolocalization studies were performed using anti-pendrin polyclonal and monoclonal antibodies. Labeling was detected on the apical surface of a subpopulation of cells within the cortical collecting ducts (CCDs) that also express the H(+)-ATPase but not aquaporin-2, indicating that pendrin is present in intercalated cells of the CCD. Furthermore, pendrin was detected exclusively within the subpopulation of intercalated cells that express the H(+)-ATPase but not the anion exchanger 1 (AE1) and that are thought to mediate bicarbonate secretion. The same distribution of pendrin was observed in mouse, rat, and human kidney. However, pendrin was not detected in kidneys from a Pds-knockout mouse. Perfused CCD tubules isolated from alkali-loaded wild-type mice secreted bicarbonate, whereas tubules from alkali-loaded Pds-knockout mice failed to secrete bicarbonate. Together, these studies indicate that pendrin is an apical anion transporter in intercalated cells of CCDs and has an essential role in renal bicarbonate secretion.
Figures



References
-
- Everett L A, Glaser B, Beck J C, Idol J R, Buchs A, Heyman M, Adawi F, Hazani E, Nassir E, Baxevanis A D, et al. Nat Genet. 1997;17:411–422. - PubMed
-
- Van Hauwe P, Everett L A, Coucke P, Scott D A, Kraft M L, Ris-Stalpers C, Bolder C, Otten B, de Vijlder J J, Dietrich N L, et al. Hum Mol Genet. 1998;7:1099–1104. - PubMed
-
- Coyle B, Reardon W, Herbrick J A, Tsui L C, Gausden E, Lee J, Coffey R, Grueters A, Grossman A, Phelps P D, et al. Hum Mol Genet. 1998;7:1105–1112. - PubMed
-
- Scott D A, Wang R, Kreman T M, Sheffield V C, Karniski L P. Nat Genet. 1999;21:440–443. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

