Total chemical synthesis of human matrix Gla protein
- PMID: 11274477
- PMCID: PMC2373974
- DOI: 10.1110/ps.44701
Total chemical synthesis of human matrix Gla protein
Abstract
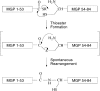
Human matrix Gla protein (MGP) is a vitamin K-dependent extracellular matrix protein that binds Ca2+ ions and that is involved in the prevention of vascular calcification. MGP is a 10.6-kD protein (84 amino acids) containing five gamma-carboxyglutamic acid (Gla) residues and one disulfide bond. Studies of the mechanism by which MGP prevents calcification of the arterial media are hampered by the low solubility of the protein (<10 microg/mL). Because of solubility problems, processing of a recombinantly expressed MGP-fusion protein chimera to obtain MGP was unsuccessful. Here we describe the total chemical synthesis of MGP by tBoc solid-phase peptide synthesis (SPPS) and native chemical ligation. Peptide Tyr1-Ala53 was synthesized on a derivatized resin yielding a C-terminal thioester group. Peptide Cys54-Lys84 was synthesized on Lys-PAM resin yielding a C-terminal carboxylic acid. Subsequent native chemical ligation of the two peptides resulted in the formation of a native peptide bond between Ala53 and Cys54. Folding of the 1-84-polypeptide chain in 3 M guanidine (pH 8) resulted in a decrease of molecular mass from 10,605 to 10,603 (ESI-MS), representing the loss of two protons because of the formation of the Cys54-Cys60 internal disulfide bond. Like native MGP, synthetic MGP had the same low solubility when brought into aqueous buffer solutions with physiological salt concentrations, confirming its native like structure. However, the solubility of MGP markedly increased in borate buffer at pH 7.4 in the absence of sodium chloride. Ca2+-binding to MGP was confirmed by analytical HPLC, on which the retention time of MGP was reduced in the presence of CaCl2. Circular dichroism studies revealed a sharp increase in alpha-helicity at 0.2 mM CaCl2 that may explain the Ca2+-dependent shift in high-pressure liquid chromatography (HPLC)-retention time of MGP. In conclusion, facile and efficient chemical synthesis in combination with native chemical ligation yielded MGP preparations that can aid in unraveling the mechanism by which MGP prevents vascular calcification.
Figures





References
-
- Braam, L.A.J.L.M., Dissel, P., Gijsbers, B.L.M.G., Spronk, H.M.H., Hamulyák, K., Soute, B.A.M., Debie, W., and Vermeer, C. 2000. Assay for human matrix Gla protein in serum. Arterioscler Thromb. Vasc. Biol. 20 1257–1261. - PubMed
-
- Chen, Y.H., Yang, J.T., and Chau, K.H. 1974. Determination of the helix and β form of proteins in aqueous solution by circular dichroism. Biochemistry 13 3350–3359. - PubMed
-
- Chen, L., O'Brian, J.P., Smith, H.S., and Liu, E. 1990. Overexpression of matrix Gla protein mRNA in malignant human breast cells, isolation by differential cDNA hybridization. Oncogene 5 1391–1395. - PubMed
-
- Chowdhury, S.K., Katta, V., and Chait, B.T. 1990. Probing conformational changes in proteins by mass spectrometry. J. Am. Chem. Soc. 112 9012–9013.
-
- Dawson, P.E., Muir, T.W., Clark-Lewis, I., Kent, S.B.H. 1994. Synthesis of proteins by native chemical ligation. Science 266 776–779. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

