Rhesus factors and ammonium: a function in efflux?
- PMID: 11276430
- PMCID: PMC138916
- DOI: 10.1186/gb-2001-2-3-reviews1010
Rhesus factors and ammonium: a function in efflux?
Abstract
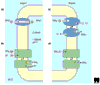
Completion of fungal, plant and human genomes paved the way to the identification of erythrocytic rhesus proteins and their kidney homologs as ammonium transporters.
Figures
References
-
- von Wiren N, Gazzarrini S, Gojon A, Frommer WB. The molecular physiology of ammonium uptake and retrieval. Curr Opin Plant Biol. 2000;3:254–261. - PubMed
-
- Kleiner D. The transport of NH3 and NH4+ across biological membranes. Biochim Biophys Acta. 1981;639:41–52. - PubMed
-
- Dubois E, Grenson M. Methylamine/ammonia uptake systems in Saccharomyces cerevisiae: multiplicity and regulation. Mol Gen Genet. 1979;175:67–76. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

