ATP-dependent adenophostin activation of inositol 1,4,5-trisphosphate receptor channel gating: kinetic implications for the durations of calcium puffs in cells
- PMID: 11279251
- PMCID: PMC2217258
- DOI: 10.1085/jgp.117.4.299
ATP-dependent adenophostin activation of inositol 1,4,5-trisphosphate receptor channel gating: kinetic implications for the durations of calcium puffs in cells
Erratum in
- J Gen Physiol 2001 Apr;117(4):369
Abstract
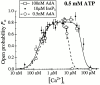
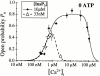
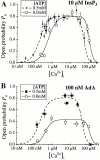
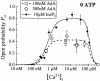
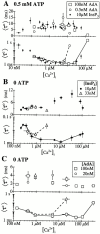
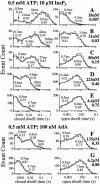
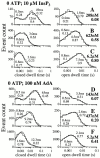
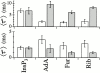
The inositol 1,4,5-trisphosphate (InsP(3)) receptor (InsP(3)R) is a ligand-gated intracellular Ca(2+) release channel that plays a central role in modulating cytoplasmic free Ca(2+) concentration ([Ca(2+)](i)). The fungal metabolite adenophostin A (AdA) is a potent agonist of the InsP(3)R that is structurally different from InsP(3) and elicits distinct calcium signals in cells. We have investigated the effects of AdA and its analogues on single-channel activities of the InsP(3)R in the outer membrane of isolated Xenopus laevis oocyte nuclei. InsP(3)R activated by either AdA or InsP(3) have identical channel conductance properties. Furthermore, AdA, like InsP(3), activates the channel by tuning Ca(2+) inhibition of gating. However, gating of the AdA-liganded InsP(3)R has a critical dependence on cytoplasmic ATP free acid concentration not observed for InsP(3)-liganded channels. Channel gating activated by AdA is indistinguishable from that elicited by InsP(3) in the presence of 0.5 mM ATP, although the functional affinity of the channel is 60-fold higher for AdA. However, in the absence of ATP, gating kinetics of AdA-liganded InsP(3)R were very different. Channel open time was reduced by 50%, resulting in substantially lower maximum open probability than channels activated by AdA in the presence of ATP, or by InsP(3) in the presence or absence of ATP. Also, the higher functional affinity of InsP(3)R for AdA than for InsP(3) is nearly abolished in the absence of ATP. Low affinity AdA analogues furanophostin and ribophostin activated InsP(3)R channels with gating properties similar to those of AdA. These results provide novel insights for interpretations of observed effects of AdA on calcium signaling, including the mechanisms that determine the durations of elementary Ca(2+) release events in cells. Comparisons of single-channel gating kinetics of the InsP(3)R activated by InsP(3), AdA, and its analogues also identify molecular elements in InsP(3)R ligands that contribute to binding and activation of channel gating.
Figures



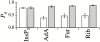
Similar articles
-
ATP regulation of type 1 inositol 1,4,5-trisphosphate receptor channel gating by allosteric tuning of Ca(2+) activation.J Biol Chem. 1999 Aug 6;274(32):22231-7. doi: 10.1074/jbc.274.32.22231. J Biol Chem. 1999. PMID: 10428789
-
ATP regulation of recombinant type 3 inositol 1,4,5-trisphosphate receptor gating.J Gen Physiol. 2001 May;117(5):447-56. doi: 10.1085/jgp.117.5.447. J Gen Physiol. 2001. PMID: 11331355 Free PMC article.
-
Rapid activation and partial inactivation of inositol trisphosphate receptors by adenophostin A.Biochem J. 2000 Dec 15;352 Pt 3(Pt 3):929-33. Biochem J. 2000. PMID: 11104705 Free PMC article.
-
Redoxing calcium from the ER.Cell. 2005 Jan 14;120(1):4-5. doi: 10.1016/j.cell.2004.12.034. Cell. 2005. PMID: 15652474 Review.
-
Inositol trisphosphate receptor Ca2+ release channels.Physiol Rev. 2007 Apr;87(2):593-658. doi: 10.1152/physrev.00035.2006. Physiol Rev. 2007. PMID: 17429043 Free PMC article. Review.
Cited by
-
Patch-clamp electrophysiology of intracellular Ca2+ channels.Cold Spring Harb Protoc. 2013 Sep 1;2013(9):787-97. doi: 10.1101/pdb.top066217. Cold Spring Harb Protoc. 2013. PMID: 24003191 Free PMC article.
-
Regulation of IP(3)R Channel Gating by Ca(2+) and Ca(2+) Binding Proteins.Curr Top Membr. 2010;66:235-72. doi: 10.1016/S1063-5823(10)66011-5. Epub 2010 Jul 25. Curr Top Membr. 2010. PMID: 22353483 Free PMC article. No abstract available.
-
IP3-mediated octopamine-induced synaptic enhancement of crayfish LG neurons.J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2012 Aug;198(8):607-15. doi: 10.1007/s00359-012-0733-2. Epub 2012 May 24. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2012. PMID: 22622466
-
Regulation of CD8 T-cell signaling, metabolism, and cytotoxic activity by extracellular lysophosphatidic acid.Immunol Rev. 2023 Aug;317(1):203-222. doi: 10.1111/imr.13208. Epub 2023 Apr 25. Immunol Rev. 2023. PMID: 37096808 Free PMC article. Review.
-
Distribution of inositol-1,4,5-trisphosphate receptor isotypes and ryanodine receptor isotypes during maturation of the rat hippocampus.Neuroscience. 2007 Dec 12;150(3):625-38. doi: 10.1016/j.neuroscience.2007.09.058. Epub 2007 Oct 3. Neuroscience. 2007. PMID: 17981403 Free PMC article.
References
-
- Baudet S.B., Hove-Madsen L., Bers D.M. How to make and use calcium-specific mini- and microelectrodes. In: Nuccitelli R., editor. A Practical Guide to the Study of Calcium in Living Cells. Academic Press; San Diego, CA: 1994. pp. 94–114. - PubMed
-
- Beecroft M.D., Marchant J.S., Riley A.M., Van Straten N.C.R., Van der Marel G.A., Van Boom J.H., Potter B.V.L., Taylor C.W. Acyclophostina ribose-modified analog of adenophostin A with high affinity for inositol 1,4,5-trisphosphate receptors and pH-dependent efficacy. Mol. Pharmacol. 1999;55:109–117. - PubMed
-
- Bezprozvanny I., Watras J., Ehrlich B.E. Bell-shaped calcium-response curves of Ins(1,4,5)P3- and calcium-gated channels from endoplasmic reticulum of cerebellum. Nature. 1991;351:751–754. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

