Does provision of an evidence-based information change public willingness to accept screening tests?
- PMID: 11281921
- PMCID: PMC5080955
- DOI: 10.1046/j.1369-6513.2000.00081.x
Does provision of an evidence-based information change public willingness to accept screening tests?
Abstract
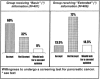
OBJECTIVE: To investigate whether the willingness of the general population to undergo a screening test of questionable effectiveness for pancreatic cancer is influenced by the quality and the extent of the information provided. DESIGN: Randomised study. SETTING: Switzerland. PARTICIPANTS: Representative sample (N=1000) of the general population aged over 20. INTERVENTIONS: Participants were randomly allocated into two groups (N=500 each), with one group to receive basic and the other extended quality of information. The information was presented in two hypothetical scenarios about implicit and explicit benefits and adverse events of the screening test. Response rates were, respectively, 80.2% (N=401) and 93.2% (N=466). MAIN OUTCOME MEASURES: Stated willingness to undergo the screening test. RESULTS: Out of the 401 participants receiving the basic information scenario, 241 (60%) stated their willingness to accept the test, as compared to the 63/466 (13.5%) exposed to the extended one (P < 0.001). After adjusting for respondent characteristics through a logistic regression model, the 'information effect', expressed in terms of odds-ratio (OR), shows that provision of additional information was related to a 91% (OR 0.09; 95CI: 0.07 - 0.13) relative reduction in the likelihood of accepting the screening test. CONCLUSION: The quality and the extent of the information provided about the implicit and explicit benefits and adverse events on hypothetical scenarios of a screening test may dramatically change the willingness of people to participate in the testing. This study suggests that provision of full information on the yield of health care interventions plays an important role in protecting the public from being exposed to procedures of questionable effectiveness.
Figures
References
-
- Entwistle VA, Sheldon TA, Sowden A, Watt IS. Evidence‐informed patient choice. Practical issues of involving patients in decisions about health care technologies. International Journal of Technology Assessment in Health Care, 1998; 14 : 212 225. - PubMed
-
- McGuire A, Henderson J, Mooney G. The economics of health care: Health care as an economic commodity London: Routledge and Kegan Paul, 1988.
-
- Bell DE. Regret in decision making under uncertainty. Operational Research, 1982; 30 : 961 981.
-
- Wolf AM. Impact of informed consent on patient interest in prostate‐specific antigen screening. Archives of Internal Medicine, 1996; 156 : 1333 1336. - PubMed
-
- Viscusi WK. Smoking: Making the Risky Decision Oxford: Oxford University Press, 1992.
LinkOut - more resources
Full Text Sources