Comparison of quantitative and qualitative PCR assays for cytomegalovirus DNA in plasma
- PMID: 11283052
- PMCID: PMC87935
- DOI: 10.1128/JCM.39.4.1334-1338.2001
Comparison of quantitative and qualitative PCR assays for cytomegalovirus DNA in plasma
Abstract
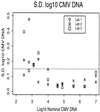
We analyzed the performance characteristics of the qualitative AMPLICOR CMV Test (Roche Molecular Systems, Pleasanton, Calif.) and quantitative COBAS AMPLICOR CMV MONITOR Test (Roche Molecular Systems) assays and compared the performance of the AMPLICOR quantitative assay with an in-house-developed cytomegalovirus (CMV) DNA PCR assay. The quantitative AMPLICOR assay was found to be more sensitive than the qualitative AMPLICOR assay. The quantitative AMPLICOR assay has a lower limit of sensitivity of 400 CMV DNA copies/ml of plasma and is linear to 50,000 CMV DNA copies/ml of plasma. Compared to the in-house PCR assay, the AMPLICOR quantitative assay gave lower viral load values at all concentrations tested, but the difference between the two assays was not consistent across the entire dynamic range of the AMPLICOR quantitative assay. At the lower end of the assay, the viral load values obtained with the in-house PCR assay were three- to fivefold (0.5 to 0.7 log units) higher than those measured with the AMPLICOR assay. At higher input concentrations, the differences between the two assays approached 10-fold. This direct comparison of the in-house assay and the quantitative AMPLICOR assay provides the ability to compare previously published in-house data with an assay widely available for future research and clinical monitoring of patients with CMV infections.
Figures

References
-
- Cope A V, Sabin C, Burroughs A, Rolles K, Griffiths P D, Emery V C. Interrelationships among quantity of human cytomegalovirus (HCMV) DNA in blood, donor-recipient serostatus, and administration of methylprednisolone as risk factors for HCMV disease following liver transplantation. J Infect Dis. 1997;176:1484–1490. - PubMed
-
- Ferreira-Gonzalez A, Fisher R A, Weymouth L A, Langley M R, Wolfe L, Wilkinson D S, Garrett C T. Clinical utility of a quantitative polymerase chain reaction for diagnosis of cytomegalovirus disease in solid organ transplant patients. Transplantation. 1999;68:991–996. - PubMed
-
- Gerna G, Zipeto D, Parea M, Revello M G, Silini E, Percivalle E, Zavattoni M, Grossi P, Milanesi G. Monitoring of human cytomegalovirus infections and ganciclovir treatment in heart transplant recipients by determination of viremia, antigenemia and DNAemia. J Infect Dis. 1991;164:488–498. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

