CD47-signal regulatory protein alpha (SIRPalpha) regulates Fcgamma and complement receptor-mediated phagocytosis
- PMID: 11283158
- PMCID: PMC2193364
- DOI: 10.1084/jem.193.7.855
CD47-signal regulatory protein alpha (SIRPalpha) regulates Fcgamma and complement receptor-mediated phagocytosis
Abstract
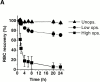
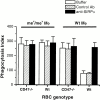
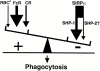
In autoimmune hemolytic anemia (AIHA), circulating red blood cells (RBCs) opsonized with autoantibody are recognized by macrophage Fcgamma and complement receptors. This triggers phagocytosis and elimination of RBCs from the circulation by splenic macrophages. We recently found that CD47 on unopsonized RBCs binds macrophage signal regulatory protein alpha (SIRPalpha), generating a negative signal that prevents phagocytosis of the unopsonized RBCs. We show here that clearance and phagocytosis of opsonized RBCs is also regulated by CD47-SIRPalpha. The inhibition generated by CD47-SIRPalpha interaction is strongly attenuated but not absent in mice with only residual activity of the phosphatase Src homology 2 domain-containing protein tyrosine phosphatase (SHP)-1, suggesting that most SIRPalpha signaling in this system is mediated by SHP-1 phosphatase activity. The macrophage phagocytic response is controlled by an integration of the inhibitory SIRPalpha signal with prophagocytic signals such as from Fcgamma and complement receptor activation. Thus, augmentation of inhibitory CD47-SIRPalpha signaling may prevent or attenuate RBC clearance in AIHA.
Figures



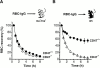

References
-
- Clynes R., Ravaetch J.V. Cytotoxic antibodies trigger inflammation through Fc receptors. Immunity. 1995;3:21–26. - PubMed
-
- Meyer D., Schiller C., Westermann J., Izui S., Hazenbos W.L.W., Verbeek J.S., Schmidt R.E., Gessner J.E. FcγRIII (CD16)-deficient mice show IgG isotype-dependent protection to experimental autoimmune hemolytic anemia. Blood. 1998;92:3997–4002. - PubMed
-
- Oldenborg P.-A., Zheleznyak A., Fang Y.-F., Lagenaur C.F., Gresham H.D., Lindberg F.P. Role of CD47 as a marker of self on red blood cells. Science. 2000;288:2051–2054. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

