Comparison of cloned Kir2 channels with native inward rectifier K+ channels from guinea-pig cardiomyocytes
- PMID: 11283229
- PMCID: PMC2278533
- DOI: 10.1111/j.1469-7793.2001.0115g.x
Comparison of cloned Kir2 channels with native inward rectifier K+ channels from guinea-pig cardiomyocytes
Abstract
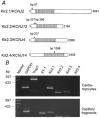
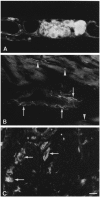
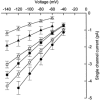
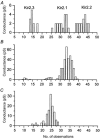
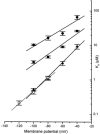
The aim of the study was to compare the properties of cloned Kir2 channels with the properties of native rectifier channels in guinea-pig (gp) cardiac muscle. The cDNAs of gpKir2.1, gpKir2.2, gpKir2.3 and gpKir2.4 were obtained by screening a cDNA library from guinea-pig cardiac ventricle. A partial genomic structure of all gpKir2 genes was deduced by comparison of the cDNAs with the nucleotide sequences derived from a guinea-pig genomic library. The cell-specific expression of Kir2 channel subunits was studied in isolated cardiomyocytes using a multi-cell RT-PCR approach. It was found that gpKir2.1, gpKir2.2 and gpKir2.3, but not gpKir2.4, are expressed in cardiomyocytes. Immunocytochemical analysis with polyclonal antibodies showed that expression of Kir2.4 is restricted to neuronal cells in the heart. After transfection in human embryonic kidney cells (HEK293) the mean single-channel conductance with symmetrical K+ was found to be 30.6 pS for gpKir2.1, 40.0 pS for gpKir2.2 and 14.2 pS for Kir2.3. Cell-attached measurements in isolated guinea-pig cardiomyocytes (n = 351) revealed three populations of inwardly rectifying K+ channels with mean conductances of 34.0, 23.8 and 10.7 pS. Expression of the gpKir2 subunits in Xenopus oocytes showed inwardly rectifying currents. The Ba2+ concentrations required for half-maximum block at -100 mV were 3.24 M for gpKir2.1, 0.51 M for gpKir2.2, 10.26 M for gpKir2.3 and 235 M for gpKir2.4. Ba2+ block of inward rectifier channels of cardiomyocytes was studied in cell-attached recordings. The concentration and voltage dependence of Ba2+ block of the large-conductance inward rectifier channels was virtually identical to that of gpKir2.2 expressed in Xenopus oocytes. Our results suggest that the large-conductance inward rectifier channels found in guinea-pig cardiomyocytes (34.0 pS) correspond to gpKir2.2. The intermediate-conductance (23.8 pS) and low-conductance (10.7 pS) channels described here may correspond to gpKir2.1 and gpKir2.3, respectively.
Figures

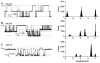
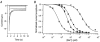

Similar articles
-
Kir2.1 encodes the inward rectifier potassium channel in rat arterial smooth muscle cells.J Physiol. 1999 Mar 15;515 ( Pt 3)(Pt 3):639-51. doi: 10.1111/j.1469-7793.1999.639ab.x. J Physiol. 1999. PMID: 10066894 Free PMC article.
-
Molecular characterization of an inwardly rectifying K+ channel from HeLa cells.J Membr Biol. 1999 Jan 1;167(1):43-52. doi: 10.1007/s002329900470. J Membr Biol. 1999. PMID: 9878074
-
Unitary conductance variation in Kir2.1 and in cardiac inward rectifier potassium channels.Biophys J. 2001 Oct;81(4):2035-49. doi: 10.1016/S0006-3495(01)75853-5. Biophys J. 2001. PMID: 11566776 Free PMC article.
-
Inward rectifier potassium channels. Cloning, expression and structure-function studies.Jpn Heart J. 1996 Sep;37(5):651-60. doi: 10.1536/ihj.37.651. Jpn Heart J. 1996. PMID: 8973378 Review.
-
Physiological role of inward rectifier K(+) channels in vascular smooth muscle cells.Pflugers Arch. 2008 Oct;457(1):137-47. doi: 10.1007/s00424-008-0512-7. Epub 2008 Apr 25. Pflugers Arch. 2008. PMID: 18437413 Review.
Cited by
-
Inward-rectifying K+ (Kir2) leak conductance dampens the excitability of lamina I projection neurons in the neonatal rat.Neuroscience. 2016 Dec 17;339:502-510. doi: 10.1016/j.neuroscience.2016.10.027. Epub 2016 Oct 14. Neuroscience. 2016. PMID: 27751963 Free PMC article.
-
Regulation of cardiac inward rectifier potassium current (I(K1)) by synapse-associated protein-97.J Biol Chem. 2010 Sep 3;285(36):28000-9. doi: 10.1074/jbc.M110.110858. Epub 2010 Jun 8. J Biol Chem. 2010. PMID: 20530486 Free PMC article.
-
Direct and specific activation of human inward rectifier K+ channels by membrane phosphatidylinositol 4,5-bisphosphate.J Biol Chem. 2010 Nov 26;285(48):37129-32. doi: 10.1074/jbc.C110.186692. Epub 2010 Oct 4. J Biol Chem. 2010. PMID: 20921230 Free PMC article.
-
Differential polyamine sensitivity in inwardly rectifying Kir2 potassium channels.J Physiol. 2006 Mar 1;571(Pt 2):287-302. doi: 10.1113/jphysiol.2005.097741. Epub 2005 Dec 22. J Physiol. 2006. PMID: 16373386 Free PMC article.
-
Effect of a neuroprotective drug, eliprodil on cardiac repolarisation: importance of the decreased repolarisation reserve in the development of proarrhythmic risk.Br J Pharmacol. 2004 Sep;143(1):152-8. doi: 10.1038/sj.bjp.0705901. Epub 2004 Aug 9. Br J Pharmacol. 2004. PMID: 15302678 Free PMC article.
References
-
- Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Analytical Biochemistry. 1987;162:156–159. - PubMed
-
- Doupnik CA, Davidson N, Lester H. The inward rectifier potassium channel family. Current Opinion in Neurobiology. 1995;5:268–277. - PubMed
-
- Fakler B, Brändle U, Glowatzki E, Weidemann S, Zenner HP, Ruppersberg JP. Strong voltage-dependent inward rectification of inward rectifier channels is caused by intracellular spermine. Cell. 1995;80:149–154. - PubMed
-
- Ficker E, Taglialatela M, Wible BA, Henley CM, Brown AM. Spermine and spermidine as gating molecules for inward rectifier K+ channels. Science. 1994;266:1068–1072. - PubMed
-
- Golding AL. Maintenance of Xenopus laevis and oocyte injection. Methods in Enzymology. 1992;207:266–279. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

