Modulation of rat erg1, erg2, erg3 and HERG K+ currents by thyrotropin-releasing hormone in anterior pituitary cells via the native signal cascade
- PMID: 11283231
- PMCID: PMC2278513
- DOI: 10.1111/j.1469-7793.2001.0143g.x
Modulation of rat erg1, erg2, erg3 and HERG K+ currents by thyrotropin-releasing hormone in anterior pituitary cells via the native signal cascade
Abstract
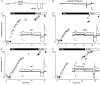
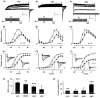
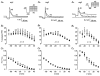
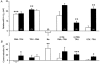
The mechanism of thyrotropin-releasing hormone (TRH)-induced ether-a-go-go-related gene (erg) K+ current modulation was investigated with the perforated-patch whole-cell technique in clonal somatomammotroph GH3/B6 cells. These cells express a small endogenous erg current known to be reduced by TRH. GH3/B6 cells were injected with cDNA coding for rat erg1, erg2, erg3 and HERG K+ channels. The corresponding erg currents were isolated with the help of the specific erg channel blockers E-4031 and dofetilide and their biophysical properties were determined. TRH (1 M) was able to significantly reduce the different erg currents. The voltage dependence of activation was shifted by 15 mV (erg1), 10 mV (erg2) and 6 mV (erg3) to more positive potentials without strongly affecting erg inactivation. TRH reduced the maximal available erg current amplitude by 12% (erg1), 13% (erg2) and 39% (erg3) and accelerated the time course of erg1 and erg2 channel deactivation, whereas erg3 deactivation kinetics were not significantly altered. The effects of TRH on HERG currents did not differ from those on its rat homologue erg1. In addition, coinjection of rat MiRP1 with HERG cDNA did not influence the TRH-induced modulation of HERG channels. Rat erg1 currents recorded in the cell-attached configuration were reduced by application of TRH to the extra-patch membrane in the majority of the experiments, confirming the involvement of a diffusible second messenger. Application of the phorbol ester phorbol 12-myristate 13-acetate (PMA; 1 M) shifted the voltage dependence of erg1 activation in the depolarizing direction, but it did not reduce the maximal current amplitude. The voltage shift could not be explained by a selective effect on protein kinase C (PKC) since the PKC inhibitor bisindolylmaleimide I did not block the effects of TRH and PMA on erg1. In addition, cholecystokinin, known to activate the phosphoinositol pathway similarly to TRH, did not significantly affect the erg1 current. Various agents interfering with different known TRH-elicited cellular responses were not able to completely mimic or inhibit the TRH effects on erg1. Tested substances included modulators of the cAMP-protein kinase A pathway, arachidonic acid, inhibitors of tyrosine kinase and mitogen-activated protein kinase, sodium nitroprusside and cytochalasin D. The results demonstrate that all three members of the erg channel subfamily are modulated by TRH in GH3/B6 cells. In agreement with previous studies on the TRH-induced modulation of the endogenous erg current in prolactin-secreting anterior pituitary cells, the TRH effects on overexpressed erg1 channels are not mediated by any of the tested signalling pathways.
Figures

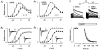



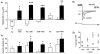
Similar articles
-
The erg-like potassium current in rat lactotrophs.J Physiol. 1999 Jul 15;518 ( Pt 2)(Pt 2):401-16. doi: 10.1111/j.1469-7793.1999.0401p.x. J Physiol. 1999. PMID: 10381588 Free PMC article.
-
Protein kinase C is necessary for recovery from the thyrotropin-releasing hormone-induced r-ERG current reduction in GH3 rat anterior pituitary cells.J Physiol. 2003 Mar 15;547(Pt 3):913-29. doi: 10.1113/jphysiol.2002.034611. Epub 2003 Jan 17. J Physiol. 2003. PMID: 12562894 Free PMC article.
-
Modulation of human erg K+ channel gating by activation of a G protein-coupled receptor and protein kinase C.J Physiol. 1998 Sep 1;511 ( Pt 2)(Pt 2):333-46. doi: 10.1111/j.1469-7793.1998.333bh.x. J Physiol. 1998. PMID: 9706014 Free PMC article.
-
Human ether-a-gogo related gene (HERG) K+ channels as pharmacological targets: present and future implications.Biochem Pharmacol. 1998 Jun 1;55(11):1741-6. doi: 10.1016/s0006-2952(98)00002-1. Biochem Pharmacol. 1998. PMID: 9714291 Review.
-
Cardiac ion channels and antihistamines: possible mechanisms of cardiotoxicity.Clin Exp Allergy. 1999 Jul;29 Suppl 3:182-9. doi: 10.1046/j.1365-2222.1999.0290s3182.x. Clin Exp Allergy. 1999. PMID: 10444235 Review.
Cited by
-
Cell type influences the molecular mechanisms involved in hormonal regulation of ERG K+ channels.Pflugers Arch. 2012 Apr;463(5):685-702. doi: 10.1007/s00424-012-1094-y. Epub 2012 Mar 14. Pflugers Arch. 2012. PMID: 22415214
-
Role of ERG1 isoforms in modulation of ERG1 channel trafficking and function.Pflugers Arch. 2010 Oct;460(5):803-12. doi: 10.1007/s00424-010-0855-8. Epub 2010 Jun 24. Pflugers Arch. 2010. PMID: 20574821 Review.
-
Expression pattern of Kv11 (Ether à-go-go-related gene; erg) K+ channels in the mouse retina.PLoS One. 2011;6(12):e29490. doi: 10.1371/journal.pone.0029490. Epub 2011 Dec 19. PLoS One. 2011. PMID: 22206018 Free PMC article.
-
Enhanced function of prefrontal serotonin 5-HT(2) receptors in a rat model of psychiatric vulnerability.J Neurosci. 2010 Sep 8;30(36):12138-50. doi: 10.1523/JNEUROSCI.3245-10.2010. J Neurosci. 2010. PMID: 20826676 Free PMC article.
-
Polyunsaturated Fatty acids modify the gating of kv channels.Front Pharmacol. 2012 Sep 10;3:163. doi: 10.3389/fphar.2012.00163. eCollection 2012. Front Pharmacol. 2012. PMID: 22973228 Free PMC article.
References
-
- Abbott GW, Sesti F, Splawski I, Buck ME, Lehmann MH, Timothy KW, Keating MT, Goldstein SAN. MiRP1 forms IKr potassium channels with HERG and is associated with cardiac arrhythmia. Cell. 1999;97:175–187. - PubMed
-
- Aragay AM, Katz A, Simon MI. The Gαq and Gα11 proteins couple the thyrotropin-releasing hormone receptor to phospholipase C in GH3 rat pituitary cells. Journal of Biological Chemistry. 1992;267:24983–24988. - PubMed
-
- Barros F, del Camino D, Pardo LA, Palomero T, Giráldez T, dela Peña P. Demonstration of an inwardly rectifying K+ current component modulated by thyrotropin-releasing hormone and caffeine in GH3 rat anterior pituitary cells. Pflügers Archiv. 1997;435:119–129. - PubMed
-
- Barros F, Delgado LM, del Camino D, de la Peña P. Characteristics and modulation by thyrotropin-releasing hormone of an inwardly rectifying K+ current in patch-perforated GH3 anterior pituitary cells. Pflügers Archiv. 1992;422:31–39. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

