S and G2 phase roles for Cdk2 revealed by inducible expression of a dominant-negative mutant in human cells
- PMID: 11283255
- PMCID: PMC86906
- DOI: 10.1128/MCB.21.8.2755-2766.2001
S and G2 phase roles for Cdk2 revealed by inducible expression of a dominant-negative mutant in human cells
Abstract
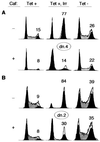
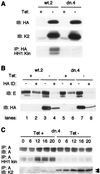
Cyclin-dependent kinase 2 (Cdk2) is essential for initiation of DNA synthesis in higher eukaryotes. Biochemical studies in Xenopus egg extracts and microinjection studies in human cells have suggested an additional function for Cdk2 in activation of Cdk1 and entry into mitosis. To further examine the role of Cdk2 in human cells, we generated stable clones with inducible expression of wild-type and dominant-negative forms of the enzyme (Cdk2-wt and Cdk2-dn, respectively). Both exogenous proteins associated efficiently with endogenous cyclins. Cdk2-wt had no apparent effect on the cell division cycle, whereas Cdk2-dn inhibited progression through several distinct stages. Cdk2-dn induction could arrest cells at the G1/S transition, as previously observed in transient expression studies. However, under normal culture conditions, Cdk2-dn induction primarily arrested cells with S and G2/M DNA contents. Several observations suggested that the latter cells were in G2 phase, prior to the onset of mitosis: these cells contained uncondensed chromosomes, low levels of cyclin B-associated kinase activity, and high levels of tyrosine-phosphorylated Cdk1. Furthermore, Cdk2-dn did not delay progression through mitosis upon release of cells from a nocodazole block. Although the G2 arrest imposed by Cdk2-dn was similar to that imposed by the DNA damage checkpoint, the former was distinguished by its resistance to caffeine. These findings provide evidence for essential functions of Cdk2 during S and G2 phases of the mammalian cell cycle.
Figures







Similar articles
-
p21waf1 can block cells at two points in the cell cycle, but does not interfere with processive DNA-replication or stress-activated kinases.Oncogene. 1998 Jan 29;16(4):431-41. doi: 10.1038/sj.onc.1201558. Oncogene. 1998. PMID: 9484832
-
Inhibition of the melanoma cell cycle and regulation at the G1/S transition by 12-O-tetradecanoylphorbol-13-acetate (TPA) by modulation of CDK2 activity.Exp Cell Res. 1995 Nov;221(1):92-102. doi: 10.1006/excr.1995.1356. Exp Cell Res. 1995. PMID: 7589260
-
Expression and subcellular localization of CDK2 and cdc2 kinases and their common partner cyclin A in thyroid epithelial cells: comparison of cyclic AMP-dependent and -independent cell cycles.J Cell Physiol. 1996 Feb;166(2):256-73. doi: 10.1002/(SICI)1097-4652(199602)166:2<256::AID-JCP3>3.0.CO;2-O. J Cell Physiol. 1996. PMID: 8591985
-
Cyclin-dependent kinases and S phase control in mammalian cells.Cell Cycle. 2003 Jul-Aug;2(4):316-24. Cell Cycle. 2003. PMID: 12851482 Review.
-
[The characterization of human cdc2 kinase and CDK2].Yakugaku Zasshi. 1993 Dec;113(12):829-46. doi: 10.1248/yakushi1947.113.12_829. Yakugaku Zasshi. 1993. PMID: 8301538 Review. Japanese.
Cited by
-
Induction of the tumor-suppressor p16(INK4a) within regenerative epithelial crypts in ulcerative colitis.Neoplasia. 2006 Jun;8(6):429-36. doi: 10.1593/neo.06169. Neoplasia. 2006. PMID: 16820088 Free PMC article.
-
MicroRNA-195 suppresses colorectal cancer cells proliferation via targeting FGF2 and regulating Wnt/β-catenin pathway.Am J Cancer Res. 2016 Nov 1;6(11):2631-2640. eCollection 2016. Am J Cancer Res. 2016. PMID: 27904776 Free PMC article.
-
Cyclin-dependent protein kinases and cell cycle regulation in biology and disease.Signal Transduct Target Ther. 2025 Jan 13;10(1):11. doi: 10.1038/s41392-024-02080-z. Signal Transduct Target Ther. 2025. PMID: 39800748 Free PMC article. Review.
-
Selenium in bone health: roles in antioxidant protection and cell proliferation.Nutrients. 2013 Jan 10;5(1):97-110. doi: 10.3390/nu5010097. Nutrients. 2013. PMID: 23306191 Free PMC article. Review.
-
Permanent cell cycle exit in G2 phase after DNA damage in normal human fibroblasts.EMBO J. 2003 Aug 1;22(15):3992-4002. doi: 10.1093/emboj/cdg387. EMBO J. 2003. PMID: 12881433 Free PMC article.
References
-
- Bunz F, Dutriaux A, Lengauer C, Waldman T, Shou S, Brown J, Sedivy J, Kinzler K, Vogelstein B. Requirement for p53 and p21 to sustain G2 arrest after DNA damage. Science. 1998;282:1497–1501. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
