Discoidin domain receptor 1 tyrosine kinase has an essential role in mammary gland development
- PMID: 11283268
- PMCID: PMC86919
- DOI: 10.1128/MCB.21.8.2906-2917.2001
Discoidin domain receptor 1 tyrosine kinase has an essential role in mammary gland development
Abstract
Various types of collagen have been identified as potential ligands for the two mammalian discoidin domain receptor tyrosine kinases, DDR1 and DDR2. Here, we used a recombinant fusion protein between the extracellular domain of DDR1 and alkaline phosphatase to detect specific receptor binding sites during mouse development. Major sites of DDR1-binding activity, indicative of ligand expression, were found in skeletal bones, the skin, and the urogenital tract. Ligand expression in the uterus during implantation and in the mammary gland during pregnancy colocalized with the expression of the DDR1 receptor. The generation of DDR1-null mice by gene targeting yielded homozygous mutant animals that were viable but smaller in size than control littermates. The majority of mutant females were unable to bear offspring due to a lack of proper blastocyst implantation into the uterine wall. When implantation did occur, the mutant females were unable to lactate. Histological analysis showed that the alveolar epithelium failed to secrete milk proteins into the lumen of the mammary gland. The lactational defect appears to be caused by hyperproliferation and abnormal branching of mammary ducts. These results suggest that DDR1 is a key mediator of the stromal-epithelial interaction during ductal morphogenesis in the mammary gland.
Figures

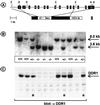




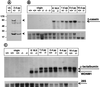
References
-
- Alves F, Vogel W, Mossie K, Millauer B, Höfler H, Ullrich A. Distinct structural characteristics of discoidin I subfamily receptor tyrosine kinases and complementary expression in human cancer. Oncogene. 1995;10:609–618. - PubMed
-
- Barker K T, Martindale J E, Mitchell P J, Kamalati T, Page M J, Phippard D J, Dale T C, Guterson B A, Crompton M R. Expression patterns of the novel receptor-like tyrosine kinase, DDR, in human breast tumors. Oncogene. 1995;10:569–575. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
