Demonstration in yeast of the function of BP-80, a putative plant vacuolar sorting receptor
- PMID: 11283336
- PMCID: PMC135539
- DOI: 10.1105/tpc.13.4.781
Demonstration in yeast of the function of BP-80, a putative plant vacuolar sorting receptor
Abstract
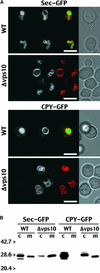
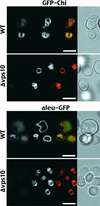
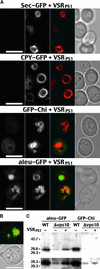
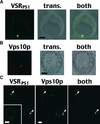
BP-80, later renamed VSR(PS-1), is a putative receptor involved in sorting proteins such as proaleurain to the lytic vacuole, with its N-terminal domain recognizing the vacuolar sorting determinant. Although all VSR(PS-1) characteristics and in vitro binding properties described so far favored its receptor function, this function remained to be demonstrated. Here, we used green fluorescent protein (GFP) as a reporter in a yeast mutant strain defective for its own vacuolar receptor, Vps10p. By expressing VSR(PS-1) together with GFP fused to the vacuolar sorting determinant of petunia proaleurain, we were able to efficiently redirect the reporter to the yeast vacuole. VSR(PS-1) is ineffective on GFP either alone or when fused with another type of plant vacuolar sorting determinant from a chitinase. The plant VSR(PS-1) therefore interacts specifically with the proaleurain vacuolar sorting determinant in vivo, and this interaction leads to the transport of the reporter protein through the yeast secretory pathway to the vacuole. This finding demonstrates VSR(PS-1) receptor function but also emphasizes the differences in the spectrum of ligands between Vps10p and its plant equivalent.
Figures


Similar articles
-
The vacuolar transport of aleurain-GFP and 2S albumin-GFP fusions is mediated by the same pre-vacuolar compartments in tobacco BY-2 and Arabidopsis suspension cultured cells.Plant J. 2008 Dec;56(5):824-39. doi: 10.1111/j.1365-313X.2008.03645.x. Epub 2008 Sep 4. Plant J. 2008. PMID: 18680561
-
Plant RMR proteins: unique vacuolar sorting receptors that couple ligand sorting with membrane internalization.FEBS J. 2011 Jan;278(1):59-68. doi: 10.1111/j.1742-4658.2010.07923.x. Epub 2010 Nov 16. FEBS J. 2011. PMID: 21078125 Review.
-
Vacuolar Sorting Determinants: Isolation and Study.Methods Mol Biol. 2018;1789:21-31. doi: 10.1007/978-1-4939-7856-4_3. Methods Mol Biol. 2018. PMID: 29916069
-
Plant Vacuoles.Annu Rev Plant Biol. 2018 Apr 29;69:123-145. doi: 10.1146/annurev-arplant-042817-040508. Epub 2018 Mar 21. Annu Rev Plant Biol. 2018. PMID: 29561663 Review.
-
BP-80 and homologs are concentrated on post-Golgi, probable lytic prevacuolar compartments.Plant Cell Physiol. 2002 Jul;43(7):726-42. doi: 10.1093/pcp/pcf085. Plant Cell Physiol. 2002. PMID: 12154135
Cited by
-
The destination for single-pass membrane proteins is influenced markedly by the length of the hydrophobic domain.Plant Cell. 2002 May;14(5):1077-92. doi: 10.1105/tpc.000620. Plant Cell. 2002. PMID: 12034898 Free PMC article.
-
In vivo intracellular pH measurements in tobacco and Arabidopsis reveal an unexpected pH gradient in the endomembrane system.Plant Cell. 2013 Oct;25(10):4028-43. doi: 10.1105/tpc.113.116897. Epub 2013 Oct 8. Plant Cell. 2013. PMID: 24104564 Free PMC article.
-
RD19, an Arabidopsis cysteine protease required for RRS1-R-mediated resistance, is relocalized to the nucleus by the Ralstonia solanacearum PopP2 effector.Plant Cell. 2008 Aug;20(8):2252-64. doi: 10.1105/tpc.108.058685. Epub 2008 Aug 15. Plant Cell. 2008. PMID: 18708476 Free PMC article.
-
Dissecting cardosin B trafficking pathways in heterologous systems.Planta. 2010 Nov;232(6):1517-30. doi: 10.1007/s00425-010-1276-9. Epub 2010 Sep 25. Planta. 2010. PMID: 20872011
-
Plant ESCRT protein ALIX coordinates with retromer complex in regulating receptor-mediated sorting of soluble vacuolar proteins.Proc Natl Acad Sci U S A. 2022 May 17;119(20):e2200492119. doi: 10.1073/pnas.2200492119. Epub 2022 May 9. Proc Natl Acad Sci U S A. 2022. PMID: 35533279 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

