A katanin-like protein regulates normal cell wall biosynthesis and cell elongation
- PMID: 11283338
- PMCID: PMC135546
A katanin-like protein regulates normal cell wall biosynthesis and cell elongation
Abstract
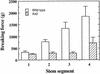
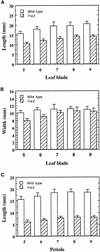
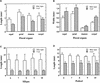
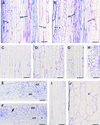
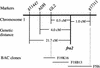
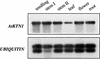
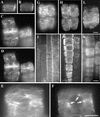
Fibers are one of the mechanical tissues that provide structural support to the plant body. To understand how the normal mechanical strength of fibers is regulated, we isolated an Arabidopsis fragile fiber (fra2) mutant defective in the mechanical strength of interfascicular fibers in the inflorescence stems. Anatomical and chemical analyses showed that the fra2 mutation caused a reduction in fiber cell length and wall thickness, a decrease in cellulose and hemicellulose contents, and an increase in lignin condensation, indicating that the fragile fiber phenotype of fra2 is a result of alterations in fiber cell elongation and cell wall biosynthesis. In addition to the effects on fibers, the fra2 mutation resulted in a remarkable reduction in cell length and an increase in cell width in all organs, which led to a global alteration in plant morphology. The FRA2 gene was shown to encode a protein with high similarity to katanin (hence FRA2 was renamed AtKTN1), a protein shown to be involved in regulating microtubule disassembly by severing microtubules. Consistent with the putative function of AtKTN1 as a microtubule-severing protein, immunolocalization demonstrated that the fra2 mutation caused delays in the disappearance of perinuclear microtubule array and in the establishment of transverse cortical microtubule array in interphase and elongating cells. Together, these results suggest that AtKTN1, a katanin-like protein, is essential not only for normal cell wall biosynthesis and cell elongation in fiber cells but also for cell expansion in all organs.
Figures





References
-
- Akin, D.E., Morrison, W.H., and Himmelsbach, D.S. (1993). Characterization of digestion residues of alfalfa and orchardgrass leaves by microscopic, spectroscopic and chemical analysis. J. Sci. Food Agric. 63, 339–347.
-
- Aloni, R. (1987). Differentiation of vascular tissues. Annu. Rev. Plant Physiol. 38, 179–204.
-
- Arioli, T., et al. (1998). Molecular analysis of cellulose biosynthesis in Arabidopsis. Science 279, 717–720. - PubMed
-
- Baskin, T.I. (2000). The cytoskeleton. In Biochemistry and Molecular Biology of Plants, B.B. Buchanan, W. Gruissem, and R.L. Jones, eds (Rockville, MD: American Society of Plant Physiologists), pp. 202–258.
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
