Auxin transport promotes Arabidopsis lateral root initiation
- PMID: 11283340
- PMCID: PMC135543
- DOI: 10.1105/tpc.13.4.843
Auxin transport promotes Arabidopsis lateral root initiation
Abstract
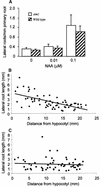
Lateral root development in Arabidopsis provides a model for the study of hormonal signals that regulate postembryonic organogenesis in higher plants. Lateral roots originate from pairs of pericycle cells, in several cell files positioned opposite the xylem pole, that initiate a series of asymmetric, transverse divisions. The auxin transport inhibitor N-1-naphthylphthalamic acid (NPA) arrests lateral root development by blocking the first transverse division(s). We investigated the basis of NPA action by using a cell-specific reporter to demonstrate that xylem pole pericycle cells retain their identity in the presence of the auxin transport inhibitor. However, NPA causes indoleacetic acid (IAA) to accumulate in the root apex while reducing levels in basal tissues critical for lateral root initiation. This pattern of IAA redistribution is consistent with NPA blocking basipetal IAA movement from the root tip. Characterization of lateral root development in the shoot meristemless1 mutant demonstrates that root basipetal and leaf acropetal auxin transport activities are required during the initiation and emergence phases, respectively, of lateral root development.
Figures






References
-
- Beeckman, T., and Engler, G. (1994). An easy technique for the clearing of histochemically stained plant tissue. Plant Mol. Biol. Rep. 12, 37–42.
-
- Blakely, L.M., Durham, M., Evans, T.A., and Blakely, R.M. (1982). Experimental studies on lateral root formation in radish seedling roots. I. General methods, developmental stages, and spontaneous formation of laterals. Bot. Gaz. 143, 341–352.
-
- Casero, P.J., Casimiro, I., Rodríguez-Gallardo, L., Martín-Partido, G., and Lloret, P.G. (1993). Lateral root initiation by means of asymmetrical transversal divisions of the pericycle cells in adventitious roots of Allium cepa. Protoplasma 176, 138–144.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

