The TRANSPARENT TESTA12 gene of Arabidopsis encodes a multidrug secondary transporter-like protein required for flavonoid sequestration in vacuoles of the seed coat endothelium
- PMID: 11283341
- PMCID: PMC135529
- DOI: 10.1105/tpc.13.4.853
The TRANSPARENT TESTA12 gene of Arabidopsis encodes a multidrug secondary transporter-like protein required for flavonoid sequestration in vacuoles of the seed coat endothelium
Abstract
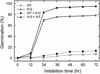
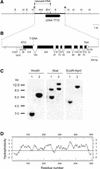
Phenolic compounds that are present in the testa interfere with the physiology of seed dormancy and germination. We isolated a recessive Arabidopsis mutant with pale brown seeds, transparent testa12 (tt12), from a reduced seed dormancy screen. Microscopic analysis of tt12 developing and mature testas revealed a strong reduction of proanthocyanidin deposition in vacuoles of endothelial cells. Double mutants with tt12 and other testa pigmentation mutants were constructed, and their phenotypes confirmed that tt12 was affected at the level of the flavonoid biosynthetic pathway. The TT12 gene was cloned and found to encode a protein with similarity to prokaryotic and eukaryotic secondary transporters with 12 transmembrane segments, belonging to the MATE (multidrug and toxic compound extrusion) family. TT12 is expressed specifically in ovules and developing seeds. In situ hybridization localized its transcript in the endothelium layer, as expected from the effect of the tt12 mutation on testa flavonoid pigmentation. The phenotype of the mutant and the nature of the gene suggest that TT12 may control the vacuolar sequestration of flavonoids in the seed coat endothelium.
Figures






References
-
- Albert, S., Delseny, M., and Devic, M. (1997). BANYULS, a novel negative regulator of flavonoid biosynthesis in the Arabidopsis seed coat. Plant J. 11, 289–299. - PubMed
-
- Barz, W. (1977). Degradation of polyphenols in plants and plant cell suspension cultures. Physiol. Veg. 15, 261–277.
-
- Baur, P.S., and Walkinshaw, C.H. (1974). Fine structure of tannin accumulation in callus cultures of Pinus elliotti (slash pine). Can. J. Bot. 52, 615–619.
-
- Bechtold, N., Ellis, J., and Pelletier, G. (1993). In planta Agrobacterium-mediated gene transfer by infiltration of adult Arabidopsis thaliana plants. C. R. Acad. Sci. Paris 316, 1194–1199.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

