Identification of two loci in tomato reveals distinct mechanisms for salt tolerance
- PMID: 11283342
- PMCID: PMC135536
- DOI: 10.1105/tpc.13.4.873
Identification of two loci in tomato reveals distinct mechanisms for salt tolerance
Abstract
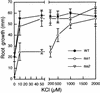
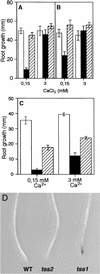
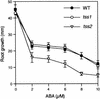
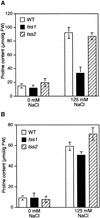
Salt stress is one of the most serious environmental factors limiting the productivity of crop plants. To understand the molecular basis for salt responses, we used mutagenesis to identify plant genes required for salt tolerance in tomato. As a result, three tomato salt-hypersensitive (tss) mutants were isolated. These mutants defined two loci and were caused by single recessive nuclear mutations. The tss1 mutant is specifically hypersensitive to growth inhibition by Na(+) or Li(+) and is not hypersensitive to general osmotic stress. The tss2 mutant is hypersensitive to growth inhibition by Na(+) or Li(+) but, in contrast to tss1, is also hypersensitive to general osmotic stress. The TSS1 locus is necessary for K(+) nutrition because tss1 mutants are unable to grow on a culture medium containing low concentrations of K(+). Increased Ca(2)+ in the culture medium suppresses the growth defect of tss1 on low K(+). Measurements of membrane potential in apical root cells were made with an intracellular microelectrode to assess the permeability of the membrane to K(+) and Na(+). K(+)-dependent membrane potential measurements indicate impaired K(+) uptake in tss1 but not tss2, whereas no differences in Na(+) uptake were found. The TSS2 locus may be a negative regulator of abscisic acid signaling, because tss2 is hypersensitive to growth inhibition by abscisic acid. Our results demonstrate that the TSS1 locus is essential for K(+) nutrition and NaCl tolerance in tomato. Significantly, the isolation of the tss2 mutant demonstrates that abscisic acid signaling is also important for salt and osmotic tolerance in glycophytic plants.
Figures






References
-
- Ashraf, M. (1994). Breeding for salinity tolerance proteins in plants. Crit. Rev. Plant Sci. 13, 17–42.
-
- Benlloch, M., Ojeda, M.A., Ramos, J., and Rodriguez-Navarro, A. (1994). Salt sensitivity and low discrimination between potassium and sodium in bean plants. Plant Soil 166, 117–123.
-
- Borsani, O., Díaz, P., and Monza, J. (1999). Proline is involved in water stress responses of Lotus corniculatus nitrogen fixing and nitrate fed plants. J. Plant Physiol. 155, 269–273.
-
- Botella, M.A., Quesada, M.A., Kononowicz, A., Bressan, R.A., Hasegawa, P.M., and Valpuesta, V. (1994). Characterization and in situ localization of a salt induced tomato peroxidase gene. Plant Mol. Biol. 25, 105–114. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

