COP9 signalosome-specific phosphorylation targets p53 to degradation by the ubiquitin system
- PMID: 11285227
- PMCID: PMC145508
- DOI: 10.1093/emboj/20.7.1630
COP9 signalosome-specific phosphorylation targets p53 to degradation by the ubiquitin system
Abstract
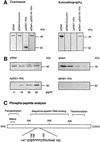
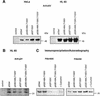
In higher eukaryotic cells, the p53 protein is degraded by the ubiquitin-26S proteasome system mediated by Mdm2 or the human papilloma virus E6 protein. Here we show that COP9 signalosome (CSN)-specific phosphorylation targets human p53 to ubiquitin-26S proteasome-dependent degradation. As visualized by electron microscopy, p53 binds with high affinity to the native CSN complex. p53 interacts via its N-terminus with CSN subunit 5/Jab1 as shown by far-western and pull-down assays. The CSN-specific phosphorylation sites were mapped to the core domain of p53 including Thr155. A phosphorylated peptide, Deltap53(145-164), specifically inhibits CSN-mediated phosphorylation and p53 degradation. Curcumin, a CSN kinase inhibitor, blocks E6-dependent p53 degradation in reticulocyte lysates. Mutation of Thr155 to valine is sufficient to stabilize p53 against E6-dependent degradation in reticulocyte lysates and to reduce binding to Mdm2. The p53T155V mutant accumulates in both HeLa and HL 60 cells and exhibits a mutant (PAb 240+) conformation. It induces the cyclin-dependent inhibitor p21. In HeLa and MCF-7 cells, inhibition of CSN kinase by curcumin or Deltap53(145-164) results in accumulation of endogenous p53.
Figures




References
-
- Banin S. et al. (1998) Enhanced phosphorylation of p53 by ATM in response to DNA damage. Science, 281, 1674–1677. - PubMed
-
- Beer-Romero P., Glass,S. and Rolfe,M. (1997) Antisense targeting of E6AP elevates p53 in HPV-infected but not in normal cells. Oncogene, 14, 595–602. - PubMed
-
- Bianchi E., Denti,S., Granata,A., Bossi,G., Geginat,J., Villa,A., Rogge,L. and Pardi,R. (2000) Integrin LFA-1 interacts with the transcriptional co-activator JAB1 to modulate AP-1 activity. Nature, 404, 617–621. - PubMed
-
- Carr A.M. (2000) Piecing together the p53 puzzle. Science, 287, 1765–1766. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

