The Vfl1 Protein in Chlamydomonas localizes in a rotationally asymmetric pattern at the distal ends of the basal bodies
- PMID: 11285274
- PMCID: PMC2185524
- DOI: 10.1083/jcb.153.1.63
The Vfl1 Protein in Chlamydomonas localizes in a rotationally asymmetric pattern at the distal ends of the basal bodies
Abstract
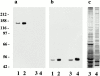
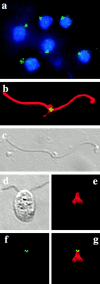
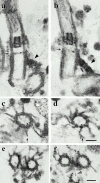
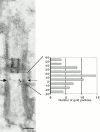
In the unicellular alga Chlamydomonas, two anterior flagella are positioned with 180 degrees rotational symmetry, such that the flagella beat with the effective strokes in opposite directions (Hoops, H.J., and G.B. Witman. 1983. J. Cell Biol. 97:902-908). The vfl1 mutation results in variable numbers and positioning of flagella and basal bodies (Adams, G.M.W., R.L. Wright, and J.W. Jarvik. 1985. J. Cell Biol. 100:955-964). Using a tagged allele, we cloned the VFL1 gene that encodes a protein of 128 kD with five leucine-rich repeat sequences near the NH(2) terminus and a large alpha-helical-coiled coil domain at the COOH terminus. An epitope-tagged gene construct rescued the mutant phenotype and expressed a tagged protein (Vfl1p) that copurified with basal body flagellar apparatuses. Immunofluorescence experiments showed that Vfl1p localized with basal bodies and probasal bodies. Immunogold labeling localized Vfl1p inside the lumen of the basal body at the distal end. Distribution of gold particles was rotationally asymmetric, with most particles located near the doublet microtubules that face the opposite basal body. The mutant phenotype, together with the localization results, suggest that Vfl1p plays a role in establishing the correct rotational orientation of basal bodies. Vfl1p is the first reported molecular marker of the rotational asymmetry inherent to basal bodies.
Figures




References
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

