Regulation of Op18 during spindle assembly in Xenopus egg extracts
- PMID: 11285281
- PMCID: PMC2185528
- DOI: 10.1083/jcb.153.1.149
Regulation of Op18 during spindle assembly in Xenopus egg extracts
Abstract
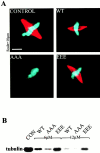
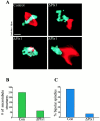
Oncoprotein 18 (Op18) is a microtubule-destabilizing protein that is negatively regulated by phosphorylation. To evaluate the role of the three Op18 phosphorylation sites in Xenopus (Ser 16, 25, and 39), we added wild-type Op18, a nonphosphorylatable triple Ser to Ala mutant (Op18-AAA), and to mimic phosphorylation, a triple Ser to Glu mutant (Op18-EEE) to egg extracts and monitored spindle assembly. Op18-AAA dramatically decreased microtubule length and density, while Op18-EEE did not significantly affect spindle microtubules. Affinity chromatography with these proteins revealed that the microtubule-destabilizing activity correlated with the ability of Op18 to bind tubulin. Since hyperphosphorylation of Op18 is observed upon addition of mitotic chromatin to extracts, we reasoned that chromatin-associated proteins might play a role in Op18 regulation. We have performed a preliminary characterization of the chromatin proteins recruited to DNA beads, and identified the Xenopus polo-like kinase Plx1 as a chromatin-associated kinase that regulates Op18 phosphorylation. Depletion of Plx1 inhibits chromatin-induced Op18 hyperphosphorylation and spindle assembly in extracts. Therefore, Plx1 may promote microtubule stabilization and spindle assembly by inhibiting Op18.
Figures





References
-
- Andersen S. Spindle assembly and the art of regulating microtubule dynamics by MAPs and Stathmin/Op18. Trends Cell Biol. 2000;10:261–267. - PubMed
-
- Andersen S.S.L., Ashford A.J., Tournebize R., Gavet O., Sobel A., Hyman A.A., Karsenti E. Mitotic chromatin regulates phosphorylation of Stathmin/Op18. Nature. 1997;389:640–643. - PubMed
-
- Arnaud L., Pines J., Nigg E.A. GFP tagging reveals human Polo-like kinase 1 at the kinetochore/centromere region of mitotic chromosomes. Chromosoma. 1998;107:424–429. - PubMed
-
- Belmont L.D., Mitchison T.J. Identification of a protein that interacts with tubulin dimers and increases the catastrophe rate of microtubules. Cell. 1996;84:623–631. - PubMed

