Nucleolar components involved in ribosome biogenesis cycle between the nucleolus and nucleoplasm in interphase cells
- PMID: 11285283
- PMCID: PMC2185520
- DOI: 10.1083/jcb.153.1.169
Nucleolar components involved in ribosome biogenesis cycle between the nucleolus and nucleoplasm in interphase cells
Abstract
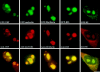
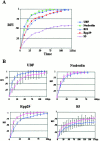
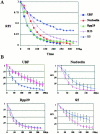
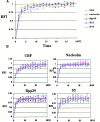
We examined the mobilities of nucleolar components that act at various steps of the ribosome biogenesis pathway. Fluorescence recovery after photobleaching (FRAP) and fluorescence loss in photobleaching (FLIP) analyses demonstrate that factors involved in rRNA transcription (upstream-binding factor [UBF]), processing (nucleolin, fibrillarin, and RNase MRP subunits, Rpp29), and ribosome assembly (B23) exchange rapidly between the nucleoplasm and nucleolus. In contrast, the mobilities of ribosomal subunit proteins (S5, L9) are much slower. Selective inhibition of RNA polymerase I transcription does not prevent the exchanges but influences the rates of exchange differentially for different nucleolar components. These findings suggest that the rapid exchange of nucleolar components between the nucleolus and nucleoplasm may represent a new level of regulation for rRNA synthesis. The different dynamic properties of proteins involved in different steps of ribosome biogenesis imply that the nucleolar association of these proteins is due to their specific functional roles rather than simply their specific nucleolar-targeting events.
Figures


References
-
- Busch H., Smetana K. Nucleoli of tumor cells. In: Busch H., editor. The Nucleolus. Academic Press; New York: 1970.
-
- Carmo-Fonseca M., Mendes-Soares L., Campos I. To be or not to be in the nucleolus. Nat. Cell Biol. 2000;2:E107–E112. - PubMed
-
- Chan Y.L., Wool I.G. The primary structure of rat ribosomal protein S6. J. Biol. Chem. 1988;263:2891–2896. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

