A novel mouse model of lipotoxic cardiomyopathy
- PMID: 11285300
- PMCID: PMC199569
- DOI: 10.1172/JCI10947
A novel mouse model of lipotoxic cardiomyopathy
Abstract
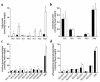
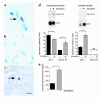
Inherited and acquired cardiomyopathies are associated with marked intracellular lipid accumulation in the heart. To test the hypothesis that mismatch between myocardial fatty acid uptake and utilization leads to the accumulation of cardiotoxic lipid species, and to establish a mouse model of metabolic cardiomyopathy, we generated transgenic mouse lines that overexpress long-chain acyl-CoA synthetase in the heart (MHC-ACS). This protein plays an important role in vectorial fatty acid transport across the plasma membrane. MHC-ACS mice demonstrate cardiac-restricted expression of the transgene and marked cardiac myocyte triglyceride accumulation. Lipid accumulation is associated with initial cardiac hypertrophy, followed by the development of left-ventricular dysfunction and premature death. Terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling staining and cytochrome c release in transgenic hearts suggest that cardiac myocyte death occurs, in part, by lipid-induced programmed cell death. Taken together, our data demonstrate that fatty acid uptake/utilization mismatch in the heart leads to accumulation of lipid species toxic to cardiac myocytes. This novel mouse model will provide insight into the role of perturbations in myocardial lipid metabolism in the pathogenesis of inherited and acquired forms of heart failure.
Figures







References
-
- Neely JR, Rovetto MJ, Oram JF. Myocardial utilization of carbohydrate and lipids. Prog Cardiovasc Dis. 1972;15:289–329. - PubMed
-
- van der Vusse GJ, Glatz JF, Stam HC, Reneman RS. Fatty acid homeostasis in the normoxic and ischemic heart. Physiol Rev. 1992;72:881–940. - PubMed
-
- Richieri GV, Kleinfeld AM. Unbound free fatty acid levels in human serum. J Lipid Res. 1995;36:229–240. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

