The regulatory role of DR4 in a spontaneous diabetes DQ8 transgenic model
- PMID: 11285306
- PMCID: PMC199575
- DOI: 10.1172/JCI11708
The regulatory role of DR4 in a spontaneous diabetes DQ8 transgenic model
Abstract
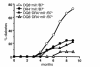
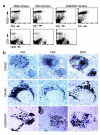
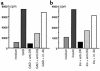
MHC class II molecules are critical determinants of genetic susceptibility to human type 1 diabetes. In patients, the most common haplotype contains the DRA1*0101-DRB1*0401 (DR4) and DQA1*0301-DQB1*0302 (DQ8) loci. To assess directly the relative roles of HLA-DQ8 and DR4 for diabetes development in vivo, we generated C57BL/6 transgenic mice that lack endogenous mouse MHC class II molecules but express HLA-DQ8 and/or DR4. Neither HLA-DQ nor HLA-DR transgenic mice developed insulitis or spontaneous diabetes. However, when they were crossed to transgenic mice (C57BL/6) expressing the B7.1 costimulatory molecules on pancreatic beta cells that do not normally develop diabetes, T cells from these double transgenic mice were no longer tolerant to islet autoantigens. The majority of DQ8/RIP-B7 mice developed spontaneous diabetes, whereas only 25% of DR4/RIP-B7 mice did so. Interestingly, when DQ8 and DR4 were coexpressed (DQ8DR4/RIP-B7), only 23% of these mice developed diabetes, an incidence indistinguishable from the DR4/RIP-B7 mice. T cells from both DR4/RIP-B7 and DQ8DR4/RIP-B7 mice, unlike those from DQ8/RIP-B7 mice, exhibited a Th2-like phenotype. Thus, the expression of DR4 appeared to downregulate DQ8-restricted autoreactive T cells in DQ8DR4/RIP-B7 mice. Our data suggest that although both DQ8 and DR4 can promote spontaneous diabetes in mice with a non-autoimmune-prone genetic background, the diabetogenic effect of the DQ8 allele is much greater, whereas DR4 expression downregulates the diabetogenic effect of DQ8, perhaps by enhancing Th2-like immune responses.
Figures


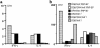
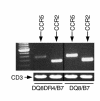
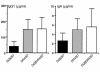
Comment in
-
DR, DQ, and you: MHC alleles and autoimmunity.J Clin Invest. 2001 Apr;107(7):795-6. doi: 10.1172/JCI12634. J Clin Invest. 2001. PMID: 11285296 Free PMC article. Review. No abstract available.
References
-
- Davies JL, et al. A genome-wide search for human type 1 diabetes susceptibility genes. Nature. 1994;371:130–136. - PubMed
-
- Tisch R, McDevitt H. Insulin-dependent diabetes mellitus. Cell. 1996;85:291–297. - PubMed
-
- Todd JA, Farrall M. Panning for gold: genome-wide scanning for linkage in type 1 diabetes. Hum Mol Genet. 1996;5:1443–1448. - PubMed
-
- Miller BJ, Appel MC, O’Neil JJ, Wicker LS. Both the Lyt-2+ and L3T4+ T cell subsets are required for the transfer of diabetes in nonobese diabetic mice. J Immunol. 1988;140:52–58. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

