Immunohistochemical and functional correlations of renal cyclooxygenase-2 in experimental diabetes
- PMID: 11285308
- PMCID: PMC199567
- DOI: 10.1172/JCI10228
Immunohistochemical and functional correlations of renal cyclooxygenase-2 in experimental diabetes
Abstract
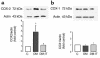
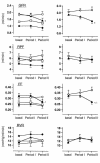
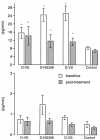
Prostaglandins (PGs) generated by the enzyme cyclooxygenase (COX) have been implicated in the pathological renal hemodynamics and structural alterations in diabetes mellitus, but the role of individual COX isoenzymes in diabetic nephropathy remains unknown. We explored COX-1 and COX-2 expression and hemodynamic responses to the COX-1 inhibitor valeryl salicylate (VS) or the COX-2 inhibitor NS398 in moderately hyperglycemic, streptozotocin-diabetic (D) and control (C) rats. Immunoreactive COX-2 was increased in D rats compared with C rats and normalized by improved glycemic control. Acute systemic administration of NS398 induced no significant changes in mean arterial pressure and renal plasma flow in either C or D rats but reduced glomerular filtration rate in D rats, resulting in a decrease in filtration fraction. VS had no effect on renal hemodynamics in D rats. Both inhibitors decreased urinary excretion of PGE(2). However, only NS398 reduced excretion of thromboxane A(2). In conclusion, we documented an increase in renal cortical COX-2 protein expression associated with a different renal hemodynamic response to selective systemic COX-2 inhibition in D as compared with C animals, indicating a role of COX-2-derived PG in pathological renal hemodynamic changes in diabetes.
Figures

References
-
- Esmatjes E, et al. Renal hemodynamic abnormalities in patients with short term insulin-dependent diabetes mellitus. J Clin Endocrinol Metab. 1985;60:1231–1236. - PubMed
-
- Hommel E, et al. Effects of indomethacin on kidney function in type 1 (insulin-dependent) diabetic patients with nephropathy. Diabetologia. 1987;30:78–81. - PubMed
-
- Gambardella S, et al. Renal hemodynamics and urinary excretion of 6-keto-prostaglandin F1 alpha and thromboxane B2 in newly diagnosed type 1 diabetic patients. Diabetes. 1988;37:1044–1048. - PubMed
-
- Viberti GC, Benigni A, Bognetti E, Remuzzi G, Wiseman MJ. Glomerular hyperfiltration and urinary prostaglandins in type 1 diabetes mellitus. Diabet Med. 1989;6:219–223. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

