Adeno-associated virus type 2-mediated gene transfer: altered endocytic processing enhances transduction efficiency in murine fibroblasts
- PMID: 11287557
- PMCID: PMC114153
- DOI: 10.1128/JVI.75.9.4080-4090.2001
Adeno-associated virus type 2-mediated gene transfer: altered endocytic processing enhances transduction efficiency in murine fibroblasts
Abstract
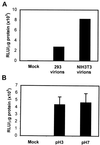
Adeno-associated virus type 2 (AAV) is a single-stranded-DNA-containing, nonpathogenic human parvovirus that is currently in use as a vector for human gene therapy. However, the transduction efficiency of AAV vectors in different cell and tissue types varies widely. In addition to the lack of expression of the viral receptor and coreceptors and the rate-limiting viral second-strand DNA synthesis, which have been identified as obstacles to AAV-mediated transduction, we have recently demonstrated that impaired intracellular trafficking of AAV inhibits high-efficiency transduction of the murine fibroblast cell line, NIH 3T3 (J. Hansen, K. Qing, H. J. Kwon, C. Mah, and A. Srivastava, J. Virol. 74:992-996, 2000). In this report, we document that escape of AAV from the endocytic pathway in NIH 3T3 cells is not limited but processing within endosomes is impaired compared with that observed in the highly permissive human cell line 293. While virions were found in both early and late endosomes or lysosomes of infected 293 cells, they were localized predominantly to the early endosomes in NIH 3T3 cells. Moreover, treatment of cells with bafilomycin A1 (Baf), an inhibitor of the vacuolar H(+)-ATPase and therefore of endosomal-lysosomal acidification, decreased the transduction of 293 cells with a concomitant decrease in nuclear trafficking of AAV but had no effect on NIH 3T3 cells. However, after exposure of NIH 3T3 cells to hydroxyurea (HU), a compound known to increase AAV-mediated transduction in general, virions were detected in late endosomes and lysosomes, and these cells became sensitive to Baf-mediated inhibition of transduction. Thus, HU treatment overcomes defective endocytic processing of AAV in murine fibroblasts. These studies provide insights into the underlying mechanisms of intracellular trafficking of AAV in different cell types, which has implications in the optimal use of AAV as vectors in human gene therapy.
Figures




Similar articles
-
Adeno-associated virus type 2-mediated gene transfer: role of cellular FKBP52 protein in transgene expression.J Virol. 2001 Oct;75(19):8968-76. doi: 10.1128/JVI.75.19.8968-8976.2001. J Virol. 2001. PMID: 11533160 Free PMC article.
-
Impaired intracellular trafficking of adeno-associated virus type 2 vectors limits efficient transduction of murine fibroblasts.J Virol. 2000 Jan;74(2):992-6. doi: 10.1128/jvi.74.2.992-996.2000. J Virol. 2000. PMID: 10623762 Free PMC article.
-
Impaired nuclear transport and uncoating limit recombinant adeno-associated virus 2 vector-mediated transduction of primary murine hematopoietic cells.Hum Gene Ther. 2004 Dec;15(12):1207-18. doi: 10.1089/hum.2004.15.1207. Hum Gene Ther. 2004. PMID: 15684697
-
Intracellular trafficking of adeno-associated viral vectors.Gene Ther. 2005 Jun;12(11):873-80. doi: 10.1038/sj.gt.3302527. Gene Ther. 2005. PMID: 15829993 Review.
-
[Gene transfer using adeno-associated virus (AAV) vectors].Nihon Rinsho. 1997 Aug;55(8):2156-9. Nihon Rinsho. 1997. PMID: 9284438 Review. Japanese.
Cited by
-
Application of doxorubicin-induced rAAV2-p53 gene delivery in combined chemotherapy and gene therapy for hepatocellular carcinoma.Cancer Biol Ther. 2008 Feb;7(2):303-9. doi: 10.4161/cbt.7.2.5333. Epub 2007 Nov 21. Cancer Biol Ther. 2008. PMID: 18059187 Free PMC article.
-
Effect of bortezomib on the efficacy of AAV9.SERCA2a treatment to preserve cardiac function in a rat pressure-overload model of heart failure.Gene Ther. 2014 Apr;21(4):379-386. doi: 10.1038/gt.2014.7. Epub 2014 Feb 27. Gene Ther. 2014. PMID: 24572786 Free PMC article.
-
Long-distance axonal transport of AAV9 is driven by dynein and kinesin-2 and is trafficked in a highly motile Rab7-positive compartment.Mol Ther. 2014 Mar;22(3):554-566. doi: 10.1038/mt.2013.237. Epub 2013 Oct 8. Mol Ther. 2014. PMID: 24100640 Free PMC article.
-
Distinct classes of proteasome-modulating agents cooperatively augment recombinant adeno-associated virus type 2 and type 5-mediated transduction from the apical surfaces of human airway epithelia.J Virol. 2004 Mar;78(6):2863-74. doi: 10.1128/jvi.78.6.2863-2874.2004. J Virol. 2004. PMID: 14990705 Free PMC article.
-
Existence of transient functional double-stranded DNA intermediates during recombinant AAV transduction.Proc Natl Acad Sci U S A. 2007 Aug 7;104(32):13104-9. doi: 10.1073/pnas.0702778104. Epub 2007 Jul 30. Proc Natl Acad Sci U S A. 2007. PMID: 17664425 Free PMC article.
References
-
- Alexander I E, Russell D W, Spence A M, Miller A D. Effects of gamma irradiation on the transduction of dividing and nondividing cells in brain and muscle of rats by adeno-associated virus vectors. Hum Gene Ther. 1996;7:841–850. - PubMed
-
- Atchison R W, Casto B C, Hammon W M. Adenovirus-associated defective virus particles. Science. 1965;149:754–756. - PubMed
-
- Bachmann P A, Hoggan M D, Kurstak E, Melnick J L, Pereira H G, Tattersall P, Vago C. Parvoviridae: second report. Intervirology. 1979;11:248–254. - PubMed
-
- Bartlett J S, Kleinschmidt J, Boucher R C, Samulski R J. Targeted adeno-associated virus vector transduction of nonpermissive cells mediated by a bispecific F(ab′gamma)2 antibody. Nat Biotechnol. 1999;17:181–186. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

