Polyadenylation in rice tungro bacilliform virus: cis-acting signals and regulation
- PMID: 11287568
- PMCID: PMC114164
- DOI: 10.1128/JVI.75.9.4184-4194.2001
Polyadenylation in rice tungro bacilliform virus: cis-acting signals and regulation
Abstract
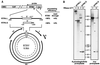
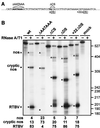
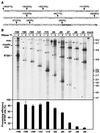
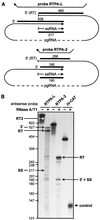
The polyadenylation signal of rice tungro bacilliform virus (RTBV) was characterized by mutational and deletion analysis. The cis-acting signals required to direct polyadenylation conformed to what is known for plant poly(A) signals in general and were very similar to those of the related cauliflower mosaic virus. Processing was directed by a canonical AAUAAA poly(A) signal, an upstream UG-rich region considerably enhanced processing efficiency, and sequences downstream of the cleavage site were not required. When present at the end of a transcription unit, the cis-acting signals for 3'-end processing were highly efficient in both monocot (rice) and dicot (Nicotiana plumbaginifolia) protoplasts. In a promoter-proximal position, as in the viral genome, the signal was also efficiently processed in rice protoplasts, giving rise to an abundant "short-stop" (SS-) RNA. The proportion of SS-RNA was considerably lower in N. plumbaginifolia protoplasts. In infected plants, SS-RNA was hardly detectable, suggesting either that SS-RNA is unstable in infected plants or that read-through of the promoter-proximal poly(A) site is very efficient. SS-RNA is readily detectable in transgenic rice plants (A. Klöti, C. Henrich, S. Bieri, X. He, G. Chen, P. K. Burkhardt, J. Wünn, P. Lucca, T. Hohn, I. Potrylus, and J. Fütterer, 1999. Plant Mol. Biol. 40:249-266), thus the absence of SS-RNA in infected plants can be attributed to poly(A) site bypass in the viral context to ensure production of the full-length pregenomic viral RNA. RTBV poly(A) site suppression thus depends both on context and the expression system; our results suggest that the circular viral minichromosome directs assembly of a transcription-processing complex with specific properties to effect read-through of the promoter-proximal poly(A) signal.
Figures


Similar articles
-
Contribution of downstream promoter elements to transcriptional regulation of the rice tungro bacilliform virus promoter.Nucleic Acids Res. 2002 Jan 15;30(2):497-506. doi: 10.1093/nar/30.2.497. Nucleic Acids Res. 2002. PMID: 11788712 Free PMC article.
-
Upstream and downstream sequence elements determine the specificity of the rice tungro bacilliform virus promoter and influence RNA production after transcription initiation.Plant Mol Biol. 1999 May;40(2):249-66. doi: 10.1023/a:1006119517262. Plant Mol Biol. 1999. PMID: 10412904
-
Efficient transcription from the rice tungro bacilliform virus promoter requires elements downstream of the transcription start site.J Virol. 1996 Dec;70(12):8411-21. doi: 10.1128/JVI.70.12.8411-8421.1996. J Virol. 1996. PMID: 8970962 Free PMC article.
-
Transcription termination and polyadenylation in retroviruses.Microbiol Rev. 1993 Sep;57(3):511-21. doi: 10.1128/mr.57.3.511-521.1993. Microbiol Rev. 1993. PMID: 7902524 Free PMC article. Review.
-
Transcriptional and translational control of gene expression in cauliflower mosaic virus.Curr Opin Genet Dev. 1992 Feb;2(1):90-6. doi: 10.1016/s0959-437x(05)80328-4. Curr Opin Genet Dev. 1992. PMID: 1633431 Review.
Cited by
-
Multifactorial analysis of terminator performance on heterologous gene expression in Physcomitrella.Plant Cell Rep. 2024 Jan 22;43(2):43. doi: 10.1007/s00299-023-03088-5. Plant Cell Rep. 2024. PMID: 38246952 Free PMC article.
-
Compilation of mRNA polyadenylation signals in Arabidopsis revealed a new signal element and potential secondary structures.Plant Physiol. 2005 Jul;138(3):1457-68. doi: 10.1104/pp.105.060541. Epub 2005 Jun 17. Plant Physiol. 2005. PMID: 15965016 Free PMC article.
-
Modeling of Genome-Wide Polyadenylation Signals in Xenopus tropicalis.Front Genet. 2019 Jul 3;10:647. doi: 10.3389/fgene.2019.00647. eCollection 2019. Front Genet. 2019. PMID: 31333724 Free PMC article.
-
Contribution of downstream promoter elements to transcriptional regulation of the rice tungro bacilliform virus promoter.Nucleic Acids Res. 2002 Jan 15;30(2):497-506. doi: 10.1093/nar/30.2.497. Nucleic Acids Res. 2002. PMID: 11788712 Free PMC article.
-
Genome level analysis of rice mRNA 3'-end processing signals and alternative polyadenylation.Nucleic Acids Res. 2008 May;36(9):3150-61. doi: 10.1093/nar/gkn158. Epub 2008 Apr 13. Nucleic Acids Res. 2008. PMID: 18411206 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

