Hepatitis B virus HBx protein activation of cyclin A-cyclin-dependent kinase 2 complexes and G1 transit via a Src kinase pathway
- PMID: 11287574
- PMCID: PMC114170
- DOI: 10.1128/JVI.75.9.4247-4257.2001
Hepatitis B virus HBx protein activation of cyclin A-cyclin-dependent kinase 2 complexes and G1 transit via a Src kinase pathway
Abstract
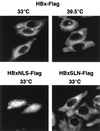
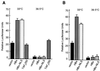
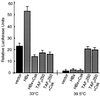
Numerous studies have demonstrated that the hepatitis B virus HBx protein stimulates signal transduction pathways and may bind to certain transcription factors, particularly the cyclic AMP response element binding protein, CREB. HBx has also been shown to promote early cell cycle progression, possibly by functionally replacing the TATA-binding protein-associated factor 250 (TAF(II)250), a transcriptional coactivator, and/or by stimulating cytoplasmic signal transduction pathways. To understand the basis for early cell cycle progression mediated by HBx, we characterized the molecular mechanism by which HBx promotes deregulation of the G0 and G1 cell cycle checkpoints in growth-arrested cells. We demonstrate that TAF(II)250 is absolutely required for HBx activation of the cyclin A promoter and for promotion of early cell cycle transit from G0 through G1. Thus, HBx does not functionally replace TAF(II)250 for transcriptional activity or for cell cycle progression, in contrast to a previous report. Instead, HBx is shown to activate the cyclin A promoter, induce cyclin A-cyclin-dependent kinase 2 complexes, and promote cycling of growth-arrested cells into G1 through a pathway involving activation of Src tyrosine kinases. HBx stimulation of Src kinases and cyclin gene expression was found to force growth-arrested cells to transit through G1 but to stall at the junction with S phase, which may be important for viral replication.
Figures




References
-
- Albright S R, Tjian R. TAFs revisited: more data reveal new twists and confirm old ideas. Gene. 2000;242:1–13. - PubMed
-
- Andrisani O, Barnabas S. The transcriptional function of the hepatitis B virus X protein and its role in hepatocarcinogenesis. Int J Oncol. 1999;15:1–7. - PubMed
-
- Barnabas S, Hai T, Andrisani O M. The hepatitis B virus X protein enhances the DNA binding potential and transcription efficacy of bZip transcription factors. J Biol Chem. 1997;272:20684–20690. - PubMed
-
- Barone M V, Courtneidge S A. Myc but not Fos rescue of PDGF signalling block caused by kinase-inactive Src. Nature. 1995;378:509–512. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

