Regulation of human immunodeficiency virus type 1 infection, beta-chemokine production, and CCR5 expression in CD40L-stimulated macrophages: immune control of viral entry
- PMID: 11287580
- PMCID: PMC114176
- DOI: 10.1128/JVI.75.9.4308-4320.2001
Regulation of human immunodeficiency virus type 1 infection, beta-chemokine production, and CCR5 expression in CD40L-stimulated macrophages: immune control of viral entry
Abstract
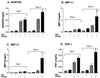
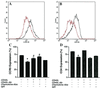
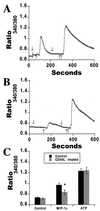
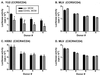
Mononuclear phagocytes (MP) and T lymphocytes play a pivotal role in the host immune response to human immunodeficiency virus type 1 (HIV-1) infection. Regulation of such immune responses can be mediated, in part, through the interaction of the T-lymphocyte-expressed molecule CD40 ligand (CD40L) with its receptor on MP, CD40. Upregulation of CD40L on CD4+ peripheral blood mononuclear cells during advanced HIV-1 disease has previously been reported. Based on this observation, we studied the influence of CD40L-CD40 interactions on MP effector function and viral regulation in vitro. We monitored productive viral infection, cytokine and beta-chemokine production, and beta-chemokine receptor expression in monocyte-derived macrophages (MDM) after treatment with soluble CD40L. Beginning 1 day after infection and continuing at 3-day intervals, treatment with CD40L inhibited productive HIV-1 infection in MDM in a dose-dependent manner. A concomitant and marked upregulation of beta-chemokines (macrophage inhibitory proteins 1alpha and 1beta and RANTES [regulated upon activation normal T-cell expressed and secreted]) and the proinflammatory cytokine tumor necrosis factor alpha (TNF-alpha) was observed in HIV-1-infected and CD40L-treated MDM relative to either infected or activated MDM alone. The addition of antibodies to RANTES or TNF-alpha led to a partial reversal of the CD40L-mediated inhibition of HIV-1 infection. Surface expression of CD4 and the beta-chemokine receptor CCR5 was reduced on MDM in response to treatment with CD40L. In addition, treatment of CCR5- and CD4-transfected 293T cells with secretory products from CD40L-stimulated MDM prior to infection with a CCR5-tropic HIV-1 reporter virus led to inhibition of viral entry. In conclusion, we demonstrate that CD40L-mediated inhibition of viral entry coincides with a broad range of MDM immune effector responses and the down-modulation of CCR5 and CD4 expression.
Figures
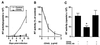




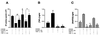
References
-
- Alkhatib G, Combadiere C, Broder C C, Feng Y, Kennedy P E, Murphy P M, Berger E A. CC CKR5: a RANTES, MIP-1α, MIP-1β receptor as a fusion cofactor for macrophage-tropic HIV-1. Science. 1996;272:1955–1958. - PubMed
-
- Alkhatib G, Locati M, Kennedy P E, Murphy P M, Berger E A. HIV-1 coreceptor activity of CCR5 and its inhibition by chemokines: independence from G protein signaling and importance of coreceptor downmodulation. Virology. 1997;234:340–348. - PubMed
-
- Bailer R T, Lee B, Montaner L J. IL-13 and TNF-alpha inhibit dual-tropic HIV-1 in primary macrophages by reduction of surface expression of CD4, chemokine receptors CCR5, CXCR4 and post-entry viral gene expression. Eur J Immunol. 2000;30:1340–1349. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

