Multiple immediate-early gene-deficient herpes simplex virus vectors allowing efficient gene delivery to neurons in culture and widespread gene delivery to the central nervous system in vivo
- PMID: 11287583
- PMCID: PMC114179
- DOI: 10.1128/JVI.75.9.4343-4356.2001
Multiple immediate-early gene-deficient herpes simplex virus vectors allowing efficient gene delivery to neurons in culture and widespread gene delivery to the central nervous system in vivo
Abstract
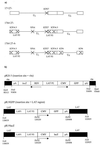
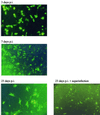
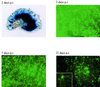
Herpes simplex virus (HSV) has several potential advantages as a vector for delivering genes to the nervous system. The virus naturally infects and remains latent in neurons and has evolved the ability of highly efficient retrograde transport from the site of infection at the periphery to the site of latency in the spinal ganglia. HSV is a large virus, potentially allowing the insertion of multiple or very large transgenes. Furthermore, HSV does not integrate into the host chromosome, removing any potential for insertional activation or inactivation of cellular genes. However, the development of HSV vectors for the central nervous system that exploit these properties has been problematical. This has mainly been due to either vector toxicity or an inability to maintain transgene expression. Here we report the development of highly disabled versions of HSV-1 deleted for ICP27, ICP4, and ICP34.5/open reading frame P and with an inactivating mutation in VP16. These viruses express only minimal levels of any of the immediate-early genes in noncomplementing cells. Transgene expression is maintained for extended periods with promoter systems containing elements from the HSV latency-associated transcript promoter (J. A. Palmer et al., J. Virol. 74:5604-5618, 2000). Unlike less-disabled viruses, these vectors allow highly effective gene delivery both to neurons in culture and to the central nervous system in vivo. Gene delivery in vivo is further enhanced by the retrograde transport capabilities of HSV. Here the vector is efficiently transported from the site of inoculation to connected sites within the nervous system. This is demonstrated by gene delivery to both the striatum and substantia nigra following striatal inoculation; to the spinal cord, spinal ganglia, and brainstem following injection into the spinal cord; and to retinal ganglion neurons following injection into the superior colliculus and thalamus.
Figures







Similar articles
-
Equine herpesvirus 1 gene 12 can substitute for vmw65 in the growth of herpes simplex virus (HSV) type 1, allowing the generation of optimized cell lines for the propagation of HSV vectors with multiple immediate-early gene defects.J Virol. 1999 Sep;73(9):7399-409. doi: 10.1128/JVI.73.9.7399-7409.1999. J Virol. 1999. PMID: 10438830 Free PMC article.
-
Comparative analysis of genomic HSV vectors for gene delivery to motor neurons following peripheral inoculation in vivo.Gene Ther. 2004 Jul;11(13):1023-32. doi: 10.1038/sj.gt.3302258. Gene Ther. 2004. PMID: 15164091
-
ICP0, ICP4, or VP16 expressed from adenovirus vectors induces reactivation of latent herpes simplex virus type 1 in primary cultures of latently infected trigeminal ganglion cells.J Virol. 2001 Jul;75(13):6143-53. doi: 10.1128/JVI.75.13.6143-6153.2001. J Virol. 2001. PMID: 11390616 Free PMC article.
-
Gene delivery using herpes simplex virus vectors.DNA Cell Biol. 2002 Dec;21(12):915-36. doi: 10.1089/104454902762053864. DNA Cell Biol. 2002. PMID: 12573050 Review.
-
Replication-defective herpes simplex virus vectors for gene transfer in vivo.Proc Natl Acad Sci U S A. 1996 Oct 15;93(21):11319-20. doi: 10.1073/pnas.93.21.11319. Proc Natl Acad Sci U S A. 1996. PMID: 8876133 Free PMC article. Review. No abstract available.
Cited by
-
Protocol Optimization for the Production of the Non-Cytotoxic JΔNI5 HSV Vector Deficient in Expression of Immediately Early Genes.Mol Ther Methods Clin Dev. 2020 Mar 17;17:612-621. doi: 10.1016/j.omtm.2020.03.014. eCollection 2020 Jun 12. Mol Ther Methods Clin Dev. 2020. PMID: 32300608 Free PMC article.
-
Herpes simplex virus type 1 induces CD83 degradation in mature dendritic cells with immediate-early kinetics via the cellular proteasome.J Virol. 2007 Jun;81(12):6326-38. doi: 10.1128/JVI.02327-06. Epub 2007 Apr 11. J Virol. 2007. PMID: 17428858 Free PMC article.
-
Herpes simplex virus ICP27 protein directly interacts with the nuclear pore complex through Nup62, inhibiting host nucleocytoplasmic transport pathways.J Biol Chem. 2012 Apr 6;287(15):12277-92. doi: 10.1074/jbc.M111.331777. Epub 2012 Feb 14. J Biol Chem. 2012. PMID: 22334672 Free PMC article.
-
Advancements in the Quest to Map, Monitor, and Manipulate Neural Circuitry.Front Neural Circuits. 2022 May 26;16:886302. doi: 10.3389/fncir.2022.886302. eCollection 2022. Front Neural Circuits. 2022. PMID: 35719420 Free PMC article. Review.
-
Defining nervous system susceptibility during acute and latent herpes simplex virus-1 infection.J Neuroimmunol. 2017 Jul 15;308:43-49. doi: 10.1016/j.jneuroim.2017.02.020. Epub 2017 Mar 8. J Neuroimmunol. 2017. PMID: 28302316 Free PMC article. Review.
References
-
- Bak I J, Markham C H, Cook M L, Stevens J G. Intraaxonal transport of herpes simplex virus in the rat central nervous system. Brain Res. 1977;136:415–429. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

