In vivo accumulation of cyclin A and cellular replication factors in autonomous parvovirus minute virus of mice-associated replication bodies
- PMID: 11287588
- PMCID: PMC114184
- DOI: 10.1128/JVI.75.9.4394-4398.2001
In vivo accumulation of cyclin A and cellular replication factors in autonomous parvovirus minute virus of mice-associated replication bodies
Abstract
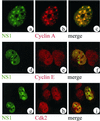
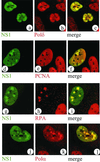
Autonomous parvovirus minute virus of mice (MVM) DNA replication is strictly dependent on cellular factors expressed during the S phase of the cell cycle. Here we report that MVM DNA replication proceeds in specific nuclear structures termed autonomous parvovirus-associated replication bodies, where components of the basic cellular replication machinery accumulate. The presence of DNA polymerases alpha and delta in these bodies suggests that MVM utilizes partially preformed cellular replication complexes for its replication. The recruitment of cyclin A points to a role for this cell cycle factor in MVM DNA replication beyond its involvement in activating the conversion of virion single-stranded DNA to the duplex replicative form.
Figures

References
-
- Brenot-Bosc F, Gupta S, Margolis R L, Fotedar R. Changes in the subcellular localization of replication initiation proteins and cell cycle proteins during G1-to S-phase transition in mammalian cells. Chromosoma. 1995;103:517–527. - PubMed
-
- Cardoso M C, Leonhardt H, Nadal-Ginard B. Reversal of terminal differentiation and control of DNA replication: cyclin A and Cdk2 specifically localize at subnuclear sites of DNA replication. Cell. 1993;74:979–992. - PubMed
-
- Christensen J. Binding of the major non-structural protein (NS1) of MVM to the large subunit of human replication protein is involved in origin unwinding. Infect Dis Rev. 2000;2:152.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

