Inflammatory responses to amyloidosis in a transgenic mouse model of Alzheimer's disease
- PMID: 11290552
- PMCID: PMC1891893
- DOI: 10.1016/S0002-9440(10)64085-0
Inflammatory responses to amyloidosis in a transgenic mouse model of Alzheimer's disease
Abstract
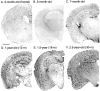
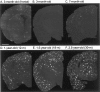
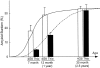
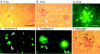
Mutations in the amyloid precursor protein (APP) and presenilin-1 and -2 genes (PS-1, -2) cause Alzheimer's disease (AD). Mice carrying both mutant genes (PS/APP) develop AD-like deposits composed of beta-amyloid (Abeta) at an early age. In this study, we have examined how Abeta deposition is associated with immune responses. Both fibrillar and nonfibrillar Abeta (diffuse) deposits were visible in the frontal cortex by 3 months, and the amyloid load increased dramatically with age. The number of fibrillar Abeta deposits increased up to the oldest age studied (2.5 years old), whereas there were less marked changes in the number of diffuse deposits in mice over 1 year old. Activated microglia and astrocytes increased synchronously with amyloid burden and were, in general, closely associated with deposits. Cyclooxygenase-2, an inflammatory response molecule involved in the prostaglandin pathway, was up-regulated in astrocytes associated with some fibrillar deposits. Complement component 1q, an immune response component, strongly colocalized with fibrillar Abeta, but was also up-regulated in some plaque-associated microglia. These results show: i) an increasing proportion of amyloid is composed of fibrillar Abeta in the aging PS/APP mouse brain; ii) microglia and astrocytes are activated by both fibrillar and diffuse Abeta; and iii) cyclooxygenase-2 and complement component 1q levels increase in response to the formation of fibrillar Abeta in PS/APP mice.
Figures





References
-
- Hardy J, Duff K, Hardy KG, Perez-Tur J, Hutton M: Genetic dissection of Alzheimer’s disease and related dementias: amyloid and its relationship to tau. Nat Neurosci 1998, 1:355-358 - PubMed
-
- Neve RL, Robakis NK: Alzheimer’s disease: a re-examination of the amyloid hypothesis. Trends Neurosci 1998, 21:15-19 - PubMed
-
- Goedert M: Tau protein and the neurofibrillary pathology of Alzheimer’s disease. Trends Neurosci 1993, 16:460-465 - PubMed
-
- Hsiao K, Chapman P, Nilsen S, Eckman C, Harigaya Y, Younkin S, Yang F, Cole G: Correlative memory deficits, Aβ elevation, and amyloid plaques in transgenic mice. Science 1996, 274:99-102 - PubMed
-
- Duff K, Eckman C, Zehr C, Yu X, Prada CM, Perez-Tur J, Hutton M, Buee L, Harigaya Y, Yager D, Morgan D, Gordon MN, Holcomb L, Refolo L, Zenk B, Hardy J, Younkin S: Increased amyloid-β42(43) in brains of mice expressing mutant presenilin 1. Nature 1996, 383:710-713 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

