Leishmaniasis: current status of vaccine development
- PMID: 11292637
- PMCID: PMC88972
- DOI: 10.1128/CMR.14.2.229-243.2001
Leishmaniasis: current status of vaccine development
Abstract
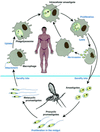
Leishmaniae are obligatory intracellular protozoa in mononuclear phagocytes. They cause a spectrum of diseases, ranging in severity from spontaneously healing skin lesions to fatal visceral disease. Worldwide, there are 2 million new cases each year and 1/10 of the world's population is at risk of infection. To date, there are no vaccines against leishmaniasis and control measures rely on chemotherapy to alleviate disease and on vector control to reduce transmission. However, a major vaccine development program aimed initially at cutaneous leishmaniasis is under way. Studies in animal models and humans are evaluating the potential of genetically modified live attenuated vaccines, as well as a variety of recombinant antigens or the DNA encoding them. The program also focuses on new adjuvants, including cytokines, and delivery systems to target the T helper type 1 immune responses required for the elimination of this intracellular organism. The availability, in the near future, of the DNA sequences of the human and Leishmania genomes will extend the vaccine program. New vaccine candidates such as parasite virulence factors will be identified. Host susceptibility genes will be mapped to allow the vaccine to be targeted to the population most in need of protection.
Figures

References
-
- Abdelhak S, Louzir H, Timm J, Blel L, Benlasfar Z, Lagranderie M, Gheorghiu M, Dellagi K, Gicquel B. Recombinant BCG expressing the leishmania surface antigen Gp63 induces protective immuity against Leishmania major infection in BALB/c mice. Microbiology. 1995;141:1585–1592. - PubMed
-
- Ahuja S S, Mummidi S, Malech H L, Ahuja S K. Human dendritic cell (DC)-based anti-infective therapy: engineering DCs to secrete functional IFN-gamma and IL-12. J Immunol. 1998;161:868–876. - PubMed
-
- Ahuja S S, Reddick R L, Sato N, Montalbo E, Kostecki V, Zhao W, Dolan M J, Melby P C, Ahuja S K. Dendritic cell (DC)-based anti-infective strategies: DCs engineered to secrete IL-12 are a potent vaccine in a murine model of an intracellular infection. J Immunol. 1999;163:3890–3897. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

