Increased immunogenicity and induction of class switching by conjugation of complement C3d to pneumococcal serotype 14 capsular polysaccharide
- PMID: 11292721
- PMCID: PMC98257
- DOI: 10.1128/IAI.69.5.3031-3040.2001
Increased immunogenicity and induction of class switching by conjugation of complement C3d to pneumococcal serotype 14 capsular polysaccharide
Abstract
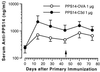
Previous studies have demonstrated an adjuvant effect for the C3d fragment of complement C3 when coupled to T-dependent protein antigens. In this study, we examined the antibody response to covalent conjugates of C3d and a T-independent antigen, the capsular polysaccharide of serotype 14 Streptococcus pneumoniae (PPS14). We prepared a conjugate of mouse C3d and PPS14 and compared its immunogenicity with that of a conjugate of PPS14 and ovalbumin (OVA). When BALB/c mice were immunized with PPS14-C3d, there was a significant increase in serum anti-PPS14 concentrations compared with either native PPS14 or control PPS14-glycine conjugates. This was accompanied by a switch in anti-PPS14 from predominantly immunoglobulin M (IgM) to IgG1 by day 25 following primary immunization. Following secondary immunization with PPS14-C3d, there was a marked booster response and a further increase in the ratio of IgG1 to IgM anti-PPS14. Although the primary antibody response to the PPS14-OVA conjugate exceeded that induced by immunization with PPS14-C3d, serum anti-PPS14 concentrations after a second injection of PPS14-C3d were nearly identical to those induced by secondary immunization with PPS14-OVA. Experiments with athymic nude mice suggested that T cells were not required for the adjuvant effect of C3d on the primary immune response to PPS14 but were necessary for enhancement of the memory response after a second injection of PPS14-C3d. These studies show that the adjuvant effects of C3d extend to T-independent antigens as well as T-dependent antigens. As a means of harnessing the adjuvant potential of the innate immune system, C3d conjugates may prove useful as a component of vaccines against encapsulated bacteria.
Figures



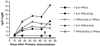
References
-
- Braley-Mullen H. Secondary IgG responses to type III pneumococcal polysaccharide. I. Kinetics and antigen requirements. J Immunol. 1975;115:1194–1198. - PubMed
-
- Brown E J. Interaction of gram-positive microorganisms with complement. Curr Top Microbiol Immunol. 1985;121:159–187. - PubMed
-
- Buchanan R M, Arulanandam B P, Metzger D W. IL-12 enhances the antibody responses to T-independent polysaccharide vaccines in the absence of T and NK cells. J Immunol. 1998;161:5525–5533. - PubMed
-
- Carter R H, Spycher M O, Ng Y C, Hoffman R, Fearon D T. Synergistic interaction between complement receptor 2 and membrane IgM on B lymphocytes. J Immunol. 1988;141:457–463. - PubMed
-
- Dempsey P W, Allison M E D, Akkaraju S, Goodnow C C, Fearon D T. C3d of complement as a molecular adjuvant: bridging innate and acquired immunity. Science. 1996;271:348–350. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

