Antigenic variation of Anaplasma marginale: major surface protein 2 diversity during cyclic transmission between ticks and cattle
- PMID: 11292724
- PMCID: PMC98260
- DOI: 10.1128/IAI.69.5.3057-3066.2001
Antigenic variation of Anaplasma marginale: major surface protein 2 diversity during cyclic transmission between ticks and cattle
Abstract
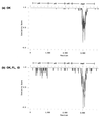
The rickettsial pathogen Anaplasma marginale expresses a variable immunodominant outer membrane protein, major surface protein 2 (MSP2), involved in antigenic variation and long-term persistence of the organism in carrier animals. MSP2 contains a central hypervariable region of about 100 amino acids that encodes immunogenic B-cell epitopes that induce variant-specific antibodies during infection. Previously, we have shown that MSP2 is encoded on a polycistronic mRNA transcript in erythrocyte stages of A. marginale and defined the structure of the genomic expression site for this transcript. In this study, we show that the same expression site is utilized in stages of A. marginale infecting tick salivary glands. We also analyzed the variability of this genomic expression site in Oklahoma strain A. marginale transmitted from in vitro cultures to cattle and between cattle and ticks. The structure of the expression site and flanking regions was conserved except for sequence that encoded the MSP2 hypervariable region. At least three different MSP2 variants were encoded in each A. marginale population. The major sequence variants did not change on passage of A. marginale between culture, acute erythrocyte stage infections, and tick salivary glands but did change during persistent infections of cattle. The variant types found in tick salivary glands most closely resembled those present in bovine blood at the time of acquisition of infection, whether infection was acquired from an acute or from a persistent rickettsemia. These variations in structure of an expression site for a major, immunoprotective outer membrane protein have important implications for vaccine development and for obtaining an improved understanding of the mechanisms of persistence of ehrlichial infections in humans, domestic animals, and reservoir hosts.
Figures





References
-
- Alleman A R, Kamper S M, Viseshakul N, Barbet A F. Analysis of the Anaplasma marginale genome by pulsed-field electrophoresis. J Gen Microbiol. 1993;139:2439–2444. - PubMed
-
- Andrew H R, Norval R A. The carrier status of sheep, cattle and African buffalo recovered from heartwater. Vet Parasitol. 1989;34:261–266. - PubMed
-
- Barbet A F, Kamper S M. The importance of mosaic genes to trypanosome survival. Parasitol Today. 1993;9:63–66. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

