Activation of extracellular signal-related protein kinases 1 and 2 of the mitogen-activated protein kinase family by lipopolysaccharide requires plasma in neutrophils from adults and newborns
- PMID: 11292734
- PMCID: PMC98270
- DOI: 10.1128/IAI.69.5.3143-3149.2001
Activation of extracellular signal-related protein kinases 1 and 2 of the mitogen-activated protein kinase family by lipopolysaccharide requires plasma in neutrophils from adults and newborns
Abstract
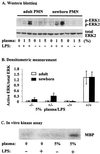
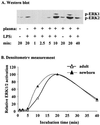
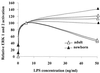
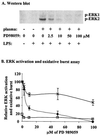
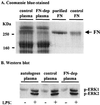
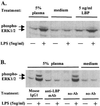
Neutrophils exposed to low concentrations of gram-negative lipopolysaccharide (LPS) become primed and have an increased oxidative response to a second stimulus (e.g., formyl-methionyl-leucyl-phenylalanine [fMLP]). In studies aimed at understanding newborn sepsis, we have shown that neutrophils of newborns are not primed in response to LPS. To further understand the processes involved in LPS-mediated priming of neutrophils, we explored the role of extracellular signal-related protein kinases (ERK 1 and 2) of the mitogen-activated protein kinase family. We found that LPS activated ERK 1 and 2 in cells of both adults and newborns and that activation was plasma dependent (maximal at > or =5%) through LPS-binding protein. Although fibronectin in plasma is required for LPS-mediated priming of neutrophils of adults assessed by fMLP-triggered oxidative burst, it was not required for LPS-mediated activation of ERK 1 and 2. LPS-mediated activation was dose and time dependent; maximal activation occurred with approximately 5 ng of LPS per ml and at 10 to 40 min. We used the inhibitor PD 98059 to study the role of ERK 1 and 2 in the LPS-primed fMLP-triggered oxidative burst. While Western blotting showed that 100 microM PD 98059 completely inhibited LPS-mediated ERK activation, oxidative response to fMLP by a chemiluminescence assay revealed that the same concentration inhibited the LPS-primed oxidative burst by only 40%. We conclude that in neutrophils, LPS-mediated activation of ERK 1 and 2 requires plasma and that this activation is not dependent on fibronectin. In addition, we found that the ERK pathway is not responsible for the lack of LPS priming in neutrophils of newborns but may be required for 40% of the LPS-primed fMLP-triggered oxidative burst in cells of adults.
Figures

References
-
- Benna J E, Han J, Park J-W, Schmid E, Ulevitch R J, Babior B M. Activation of p38 in stimulated human neutrophils: phosphorylation of the oxidase component p47phox by p38 and ERK but not by JNK. Arch Biochem Biophys. 1996;334:395–400. - PubMed
-
- Bortolussi R, Howlett S, Rajaraman K, Halperin S. Deficient priming activity of newborn cord blood-derived polymorphonuclear neutrophilic granulocytes with lipopolysaccharide and tumor necrosis factor-α triggered with formyl-methionyl-leucyl-phenylalanine. Pediatr Res. 1993;34:243–248. - PubMed
-
- Bortolussi R, Rajaraman K, Qing G, Rajaraman R. Fibronectin enhances in vitro lipopolysaccharide priming of polymorphonuclear leukocytes. Blood. 1997;89:4182–4189. - PubMed
-
- Chen Q, Lin T H, Der C J, Juliano R L. Integrin-mediated activation of mitogen-activated protein (MAP) or extracellular signal-related kinase kinase (MEK) and kinase is independent of Ras. J Biol Chem. 1996;271:18122–18127. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

