Function-based selection and characterization of base-pair polymorphisms in a promoter of Escherichia coli RNA polymerase-sigma(70)
- PMID: 11292807
- PMCID: PMC99504
- DOI: 10.1128/JB.183.9.2866-2873.2001
Function-based selection and characterization of base-pair polymorphisms in a promoter of Escherichia coli RNA polymerase-sigma(70)
Abstract
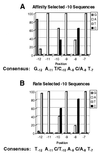
We performed two sets of in vitro selections to dissect the role of the -10 base sequence in determining the rate and efficiency with which Escherichia coli RNA polymerase-sigma(70) forms stable complexes with a promoter. We identified sequences that (i) rapidly form heparin-resistant complexes with RNA polymerase or (ii) form heparin-resistant complexes at very low RNA polymerase concentrations. The sequences selected under the two conditions differ from each other and from the consensus -10 sequence. The selected promoters have the expected enhanced binding and kinetic properties and are functionally better than the consensus promoter sequence in directing RNA synthesis in vitro. Detailed analysis of the selected promoter functions shows that each step in this multistep pathway may have different sequence requirements, meaning that the sequence of a strong promoter does not contain the optimal sequence for each step but instead is a compromise sequence that allows all steps to proceed with minimal constraint.
Figures


References
-
- Buc H, McClure W R. Kinetics of open complex formation between Escherichia coli RNA polymerase and the lac UV5 promoter. Evidence for a sequential mechanism involving three steps. Biochemistry. 1985;24:2712–2723. - PubMed
-
- Bushman F D. The bacteriophage 434 right operator. Roles of OR1, OR2 and OR3. J Mol Biol. 1993;230:28–40. - PubMed
-
- Craig M L, Suh W C, Record M T., Jr HO⋅ and DNase I probing of E sigma 70 RNA polymerase-lambda PR promoter open complexes: Mg2+ binding and its structural consequences at the transcription start site. Biochemistry. 1995;34:15624–15632. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

