Distinct modes of macrophage recognition for apoptotic and necrotic cells are not specified exclusively by phosphatidylserine exposure
- PMID: 11294896
- PMCID: PMC32276
- DOI: 10.1091/mbc.12.4.919
Distinct modes of macrophage recognition for apoptotic and necrotic cells are not specified exclusively by phosphatidylserine exposure
Abstract
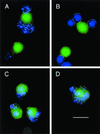
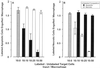
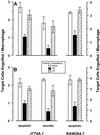
The distinction between physiological (apoptotic) and pathological (necrotic) cell deaths reflects mechanistic differences in cellular disintegration and is of functional significance with respect to the outcomes that are triggered by the cell corpses. Mechanistically, apoptotic cells die via an active and ordered pathway; necrotic deaths, conversely, are chaotic and passive. Macrophages and other phagocytic cells recognize and engulf these dead cells. This clearance is believed to reveal an innate immunity, associated with inflammation in cases of pathological but not physiological cell deaths. Using objective and quantitative measures to assess these processes, we find that macrophages bind and engulf native apoptotic and necrotic cells to similar extents and with similar kinetics. However, recognition of these two classes of dying cells occurs via distinct and noncompeting mechanisms. Phosphatidylserine, which is externalized on both apoptotic and necrotic cells, is not a specific ligand for the recognition of either one. The distinct modes of recognition for these different corpses are linked to opposing responses from engulfing macrophages. Necrotic cells, when recognized, enhance proinflammatory responses of activated macrophages, although they are not sufficient to trigger macrophage activation. In marked contrast, apoptotic cells profoundly inhibit phlogistic macrophage responses; this represents a cell-associated, dominant-acting anti-inflammatory signaling activity acquired posttranslationally during the process of physiological cell death.
Figures



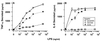
References
-
- Bellingan GJ, Caldwell H, Howie SEM, Dransfield I, Haslett C. In vivo fate of the inflammatory macrophage during the resolution of inflammation: inflammatory macrophages do not die locally, but emigrate to the draining lymph nodes. J Immunol. 1996;157:2577–2585. - PubMed
-
- Bratton DL, Fadok VA, Richter DA, Kailey JM, Guthrie LA, Henson PM. Appearance of phosphatidylserine on apoptotic cells requires calcium-mediated nonspecific flip-flop and is enhanced by loss of the aminophospholipid translocase. J Biol Chem. 1997;272:26159–26165. - PubMed
-
- Cecconi F, Alvarez-Bolado G, Meyer BI, Roth KA, Gruss P. Apaf1 (CED-4 homolog) regulates programmed cell death in mammalian development. Cell. 1998;94:727–737. - PubMed
-
- Chang M-K, Bergmark C, Laurila A, Hörkkö S, Han K-H, Friedman P, Dennis EA, Witztum JL. Monoclonal antibodies against oxidized low-density lipoprotein bind to apoptotic cells and inhibit their phagocytosis by elicited macrophages: evidence that oxidation-specific epitopes mediate macrophage recognition. Proc Natl Acad Sci USA. 1999;96:6353–6358. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

