Cell cycle-dependent changes in microtubule dynamics in living cells expressing green fluorescent protein-alpha tubulin
- PMID: 11294900
- PMCID: PMC32280
- DOI: 10.1091/mbc.12.4.971
Cell cycle-dependent changes in microtubule dynamics in living cells expressing green fluorescent protein-alpha tubulin
Abstract
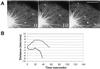
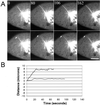
LLCPK-1 cells were transfected with a green fluorescent protein (GFP)-alpha tubulin construct and a cell line permanently expressing GFP-alpha tubulin was established (LLCPK-1alpha). The mitotic index and doubling time for LLCPK-1alpha were not significantly different from parental cells. Quantitative immunoblotting showed that 17% of the tubulin in LLCPK-1alpha cells was GFP-tubulin; the level of unlabeled tubulin was reduced to 82% of that in parental cells. The parameters of microtubule dynamic instability were compared for interphase LLCPK-1alpha and parental cells injected with rhodamine-labeled tubulin. Dynamic instability was very similar in the two cases, demonstrating that LLCPK-1alpha cells are a useful tool for analysis of microtubule dynamics throughout the cell cycle. Comparison of astral microtubule behavior in mitosis with microtubule behavior in interphase demonstrated that the frequency of catastrophe increased twofold and that the frequency of rescue decreased nearly fourfold in mitotic compared with interphase cells. The percentage of time that microtubules spent in an attenuated state, or pause, was also dramatically reduced, from 73.5% in interphase to 11.4% in mitosis. The rates of microtubule elongation and rapid shortening were not changed; overall dynamicity increased 3.6-fold in mitosis. Microtubule release from the centrosome and a subset of differentially stable astral microtubules were also observed. The results provide the first quantitative measurements of mitotic microtubule dynamics in mammalian cells.
Figures


References
-
- Andersen SSL. Balanced regulation of microtubule dynamics during the cell cycle: a contemporary view. BioEssays. 1999;21:53–60. - PubMed
-
- Belmont LD, Hyman AA, Sawin KE, Mitchison TJ. Real-time visualization of cell cycle dependent changes in microtubule dynamics in cytoplasmic extracts. Cell. 1990;62:579–589. - PubMed
-
- Busson S, Dujardin D, Moreau A, Dompierre J, DeMay JR. Dynein and dynactin are localized to astral microtubules and at cortical sites in mitotic epithelial cells. Curr Biol. 1998;8:541–544. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

