Roles of a fimbrin and an alpha-actinin-like protein in fission yeast cell polarization and cytokinesis
- PMID: 11294907
- PMCID: PMC32287
- DOI: 10.1091/mbc.12.4.1061
Roles of a fimbrin and an alpha-actinin-like protein in fission yeast cell polarization and cytokinesis
Abstract
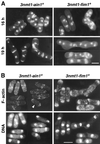
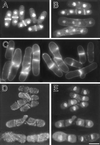
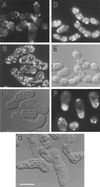
Eukaryotic cells contain many actin-interacting proteins, including the alpha-actinins and the fimbrins, both of which have actin cross-linking activity in vitro. We report here the identification and characterization of both an alpha-actinin-like protein (Ain1p) and a fimbrin (Fim1p) in the fission yeast Schizosaccharomyces pombe. Ain1p localizes to the actomyosin-containing medial ring in an F-actin-dependent manner, and the Ain1p ring contracts during cytokinesis. ain1 deletion cells have no obvious defects under normal growth conditions but display severe cytokinesis defects, associated with defects in medial-ring and septum formation, under certain stress conditions. Overexpression of Ain1p also causes cytokinesis defects, and the ain1 deletion shows synthetic effects with other mutations known to affect medial-ring positioning and/or organization. Fim1p localizes both to the cortical actin patches and to the medial ring in an F-actin-dependent manner, and several lines of evidence suggest that Fim1p is involved in polarization of the actin cytoskeleton. Although a fim1 deletion strain has no detectable defect in cytokinesis, overexpression of Fim1p causes a lethal cytokinesis defect associated with a failure to form the medial ring and concentrate actin patches at the cell middle. Moreover, an ain1 fim1 double mutant has a synthetical-lethal defect in medial-ring assembly and cell division. Thus, Ain1p and Fim1p appear to have an overlapping and essential function in fission yeast cytokinesis. In addition, protein-localization and mutant-phenotype data suggest that Fim1p, but not Ain1p, plays important roles in mating and in spore formation.
Figures
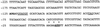





Similar articles
-
Cooperation between tropomyosin and α-actinin inhibits fimbrin association with actin filament networks in fission yeast.Elife. 2019 Jun 10;8:e47279. doi: 10.7554/eLife.47279. Elife. 2019. PMID: 31180322 Free PMC article.
-
Interactions among a fimbrin, a capping protein, and an actin-depolymerizing factor in organization of the fission yeast actin cytoskeleton.Mol Biol Cell. 2001 Nov;12(11):3515-26. doi: 10.1091/mbc.12.11.3515. Mol Biol Cell. 2001. PMID: 11694585 Free PMC article.
-
Fimbrin and tropomyosin competition regulates endocytosis and cytokinesis kinetics in fission yeast.Curr Biol. 2010 Aug 24;20(16):1415-22. doi: 10.1016/j.cub.2010.06.020. Epub 2010 Aug 12. Curr Biol. 2010. PMID: 20705466 Free PMC article.
-
Studies in fission yeast on mechanisms of cell division site placement.Cell Struct Funct. 2001 Dec;26(6):539-44. doi: 10.1247/csf.26.539. Cell Struct Funct. 2001. PMID: 11942607 Review.
-
Microtubule and actin-dependent movement of the formin cdc12p in fission yeast.Microsc Res Tech. 2000 Apr 15;49(2):161-7. doi: 10.1002/(SICI)1097-0029(20000415)49:2<161::AID-JEMT8>3.0.CO;2-2. Microsc Res Tech. 2000. PMID: 10816255 Review.
Cited by
-
Roles of the DYRK kinase Pom2 in cytokinesis, mitochondrial morphology, and sporulation in fission yeast.PLoS One. 2011;6(12):e28000. doi: 10.1371/journal.pone.0028000. Epub 2011 Dec 12. PLoS One. 2011. PMID: 22174761 Free PMC article.
-
Calpain 2 activation of P-TEFb drives megakaryocyte morphogenesis and is disrupted by leukemogenic GATA1 mutation.Dev Cell. 2013 Dec 23;27(6):607-20. doi: 10.1016/j.devcel.2013.11.013. Dev Cell. 2013. PMID: 24369834 Free PMC article.
-
Dynamic network morphology and tension buildup in a 3D model of cytokinetic ring assembly.Biophys J. 2014 Dec 2;107(11):2618-28. doi: 10.1016/j.bpj.2014.10.034. Epub 2014 Dec 2. Biophys J. 2014. PMID: 25468341 Free PMC article.
-
Actin filament bundling by fimbrin is important for endocytosis, cytokinesis, and polarization in fission yeast.J Biol Chem. 2011 Jul 29;286(30):26964-77. doi: 10.1074/jbc.M111.239004. Epub 2011 Jun 3. J Biol Chem. 2011. PMID: 21642440 Free PMC article.
-
FgFim, a key protein regulating resistance to the fungicide JS399-19, asexual and sexual development, stress responses and virulence in Fusarium graminearum.Mol Plant Pathol. 2014 Jun;15(5):488-99. doi: 10.1111/mpp.12108. Epub 2014 Jan 15. Mol Plant Pathol. 2014. PMID: 24299032 Free PMC article.
References
-
- Adams AEM, Botstein D, Drubin DG. Requirement of yeast fimbrin for actin organization and morphogenesis in vivo. Nature. 1991;354:404–408. - PubMed
-
- Alberts B, Bray D, Lewis J, Raff M, Roberts K, Watson JD. Molecular Biology of the Cell. 3rd edition. New York, New York: Garland Publishing; 1994.
-
- Arai R, Nakano K, Mabuchi I. Subcellular localization and possible function of actin, tropomyosin and actin-related protein 3 (Arp3) in the fission yeast Schizosaccharomyces pombe. Eur J Cell Biol. 1998;76:288–295. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

