Changes in organization of the endoplasmic reticulum during Xenopus oocyte maturation and activation
- PMID: 11294910
- PMCID: PMC32290
- DOI: 10.1091/mbc.12.4.1103
Changes in organization of the endoplasmic reticulum during Xenopus oocyte maturation and activation
Abstract
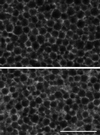
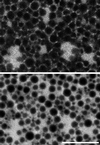
The organization of the endoplasmic reticulum (ER) in the cortex of Xenopus oocytes was investigated during maturation and activation using a green fluorescent protein chimera, immunofluorescence, and electron microscopy. Dense clusters of ER developed on the vegetal side (the side opposite the meiotic spindle) during maturation. Small clusters appeared transiently at the time of nuclear envelope breakdown, disappeared at the time of first polar body formation, and then reappeared as larger clusters in mature eggs. The appearance of the large ER clusters was correlated with an increase in releasability of Ca(2+) by IP(3). The clusters dispersed during the Ca(2+) wave at activation. Possible relationships of ER structure and Ca(2+) regulation are discussed.
Figures









Similar articles
-
Developmental expression of the inositol 1,4,5-trisphosphate receptor and structural changes in the endoplasmic reticulum during oogenesis and meiotic maturation of Xenopus laevis.Dev Biol. 1997 Feb 15;182(2):228-39. doi: 10.1006/dbio.1996.8479. Dev Biol. 1997. PMID: 9070324
-
Developmental changes in the distribution of the endoplasmic reticulum and inositol 1,4,5-trisphosphate receptors and the spatial pattern of Ca2+ release during maturation of hamster oocytes.Dev Biol. 1995 Aug;170(2):594-606. doi: 10.1006/dbio.1995.1239. Dev Biol. 1995. PMID: 7649386
-
Cell cycle-dependent regulation of structure of endoplasmic reticulum and inositol 1,4,5-trisphosphate-induced Ca2+ release in mouse oocytes and embryos.Mol Biol Cell. 2003 Jan;14(1):288-301. doi: 10.1091/mbc.e02-07-0431. Mol Biol Cell. 2003. PMID: 12529444 Free PMC article.
-
Ca2+ signaling differentiation during oocyte maturation.J Cell Physiol. 2007 Nov;213(2):331-40. doi: 10.1002/jcp.21194. J Cell Physiol. 2007. PMID: 17620315 Review.
-
Structure and function of the egg cortex from oogenesis through fertilization.Dev Biol. 2002 Jan 1;241(1):1-23. doi: 10.1006/dbio.2001.0474. Dev Biol. 2002. PMID: 11784091 Review. No abstract available.
Cited by
-
Two distinct Staufen isoforms in Xenopus are vegetally localized during oogenesis.RNA. 2004 Nov;10(11):1751-63. doi: 10.1261/rna.7450204. RNA. 2004. PMID: 15496522 Free PMC article.
-
Insemination or phosphatidic acid induces an outwardly spiraling disk of elevated Ca2+ to produce the Ca2+ wave during Xenopus laevis fertilization.Dev Biol. 2019 Apr 1;448(1):59-68. doi: 10.1016/j.ydbio.2019.01.004. Epub 2019 Jan 11. Dev Biol. 2019. PMID: 30641042 Free PMC article.
-
Involvement of the actin cytoskeleton and homotypic membrane fusion in ER dynamics in Caenorhabditis elegans.Mol Biol Cell. 2005 May;16(5):2139-53. doi: 10.1091/mbc.e04-08-0726. Epub 2005 Feb 16. Mol Biol Cell. 2005. PMID: 15716356 Free PMC article.
-
Orai1 internalization and STIM1 clustering inhibition modulate SOCE inactivation during meiosis.Proc Natl Acad Sci U S A. 2009 Oct 13;106(41):17401-6. doi: 10.1073/pnas.0904651106. Epub 2009 Sep 30. Proc Natl Acad Sci U S A. 2009. PMID: 19805124 Free PMC article.
-
The endoplasmic reticulum: structure, function and response to cellular signaling.Cell Mol Life Sci. 2016 Jan;73(1):79-94. doi: 10.1007/s00018-015-2052-6. Epub 2015 Oct 3. Cell Mol Life Sci. 2016. PMID: 26433683 Free PMC article. Review.
References
-
- Campanella C, Andreuccetti P. Ultrastructural observations on cortical endoplasmic reticulum and on residual cortical granules in the egg of Xenopus laevis. Dev Biol. 1977;56:1–10. - PubMed
-
- Campanella C, Andreuccetti P, Taddei C, Talevi R. The modifications of cortical endoplasmic reticulum during in vitro maturation of Xenopus laevis oocytes and its involvement in cortical granule exocytosis. J Exp Zool. 1984;229:283–293. - PubMed
-
- Carroll DJ, Albay DT, Terasaki M, Jaffe LA, Foltz KR. Identification of PLCgamma-dependent and -independent events during fertilization of sea urchin eggs. Dev Biol. 1999;206:232–247. - PubMed
-
- Carroll J. Na+ – Ca2+ exchange in mouse oocytes: modifications in the regulation of intracellular free Ca2+ during oocyte maturation. J Reprod Fertil. 2000;118:337–342. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

