Perinuclear, perigranular and sub-plasmalemmal mitochondria have distinct functions in the regulation of cellular calcium transport
- PMID: 11296220
- PMCID: PMC125431
- DOI: 10.1093/emboj/20.8.1863
Perinuclear, perigranular and sub-plasmalemmal mitochondria have distinct functions in the regulation of cellular calcium transport
Abstract
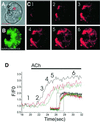
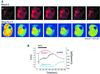
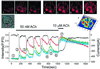
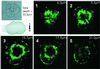
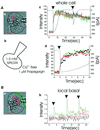
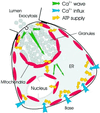
We have identified three distinct groups of mitochondria in normal living pancreatic acinar cells, located (i) in the peripheral basolateral region close to the plasma membrane, (ii) around the nucleus and (iii) in the periphery of the granular region separating the granules from the basolateral area. Three-dimensional reconstruction of confocal slices showed that the perigranular mitochondria form a barrier surrounding the whole of the granular region. Cytosolic Ca(2+) oscillations initiated in the granular area triggered mitochondrial Ca(2+) uptake mainly in the perigranular area. The most intensive uptake occurred in the mitochondria close to the apical plasma membrane. Store-operated Ca(2+) influx through the basolateral membrane caused preferential Ca(2+) uptake into sub-plasmalemmal mitochondria. The perinuclear mitochondria were activated specifically by local uncaging of Ca(2+) in the nucleus. These mitochondria could isolate nuclear and cytosolic Ca(2+) signalling. Photobleaching experiments indicated that different groups of mitochondria were not luminally connected. The three mitochondrial groups are activated independently by specific spatiotemporal patterns of cytosolic Ca(2+) signals and can therefore participate in the local regulation of Ca(2+) homeostasis and energy supply.
Figures




References
-
- Baker P.F. and Knight,D.E. (1978) Calcium-dependent exocytosis in bovine adrenal medullary cells with leaky plasma membranes. Nature, 276, 620–622. - PubMed
-
- Cancela J.M., Gerasimenko,O.V., Gerasimenko,J.V., Tepikin,A.V. and Petersen,O.H. (2000) Two different but converging messenger pathways to intracellular Ca2+ release: the roles of nicotinic acid adenine dinucleotide phosphate, cyclic ADP-ribose and inositol trisphosphate. EMBO J., 19, 2549–2557. - PMC - PubMed
-
- De Giorgi F., Lartigue,L. and Ichas,F. (2000) Electrical coupling and plasticity of the mitochondrial network. Cell Calcium, 28, 365–370. - PubMed
-
- Denton R.M. and McCormack,J.G. (1990) Ca2+ as a second messenger within mitochondria of the heart and other tissues. Annu. Rev. Physiol., 52, 451–466. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

