DNA polymerase epsilon is required for coordinated and efficient chromosomal DNA replication in Xenopus egg extracts
- PMID: 11296256
- PMCID: PMC33149
- DOI: 10.1073/pnas.081088798
DNA polymerase epsilon is required for coordinated and efficient chromosomal DNA replication in Xenopus egg extracts
Abstract
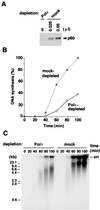
DNA polymerase epsilon (Pol epsilon) is thought to be involved in DNA replication, repair, and cell-cycle checkpoint control in eukaryotic cells. Although the requirement of other replicative DNA polymerases, DNA polymerases alpha and delta (Pol alpha and delta), for chromosomal DNA replication has been well documented by genetic and biochemical studies, the precise role, if any, of Pol epsilon in chromosomal DNA replication is still obscure. Here we show, with the use of a cell-free replication system with Xenopus egg extracts, that Xenopus Pol epsilon is indeed required for chromosomal DNA replication. In Pol epsilon-depleted extracts, the elongation step of chromosomal DNA replication is markedly impaired, resulting in significant reduction of the overall DNA synthesis as well as accumulation of small replication intermediates. Moreover, despite the decreased DNA synthesis, excess amounts of Pol alpha are loaded onto the chromatin template in Pol epsilon-depleted extracts, indicative of the failure of proper assembly of DNA synthesis machinery at the fork. These findings strongly suggest that Pol epsilon, along with Pol alpha and Pol delta, is necessary for coordinated chromosomal DNA replication in eukaryotic cells.
Figures




References
-
- Waga S, Stillman B. Annu Rev Biochem. 1998;67:721–751. - PubMed
-
- Hübscher U, Nasheuer H-P, Syväoja J E. Trends Biochem Sci. 2000;25:143–147. - PubMed
-
- Waga S, Stillman B. Nature (London) 1994;369:207–212. - PubMed
-
- Waga S, Bauer G, Stillman B. J Biol Chem. 1994;269:10923–10934. - PubMed
-
- Sugino A. Trends Biochem Sci. 1995;20:319–323. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources

