Muscle-regulated expression and determinants for neuromuscular junctional localization of the mouse RIalpha regulatory subunit of cAMP-dependent protein kinase
- PMID: 11296260
- PMCID: PMC33159
- DOI: 10.1073/pnas.081393598
Muscle-regulated expression and determinants for neuromuscular junctional localization of the mouse RIalpha regulatory subunit of cAMP-dependent protein kinase
Abstract
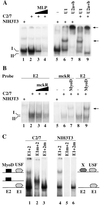
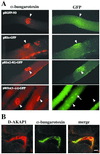
In skeletal muscle, transcription of the gene encoding the mouse type Ialpha (RIalpha) subunit of the cAMP-dependent protein kinase is initiated from the alternative noncoding first exons 1a and 1b. Here, we report that activity of the promoter upstream of exon 1a (Pa) depends on two adjacent E boxes (E1 and E2) in NIH 3T3-transfected fibroblasts as well as in intact muscle. Both basal activity and MyoD transactivation of the Pa promoter require binding of the upstream stimulating factors (USF) to E1. E2 binds either an unknown protein in a USF/E1 complex-dependent manner or MyoD. Both E2-bound proteins seem to function as repressors, but with different strengths, of the USF transactivation potential. Previous work has shown localization of the RIalpha protein at the neuromuscular junction. Using DNA injection into muscle of plasmids encoding segments of RIalpha or RIIalpha fused to green fluorescent protein, we demonstrate that anchoring at the neuromuscular junction is specific to RIalpha subunits and requires the amino-terminal residues 1-81. Mutagenesis of Phe-54 to Ala in the full-length RIalpha-green fluorescent protein template abolishes localization, indicating that dimerization of RIalpha is essential for anchoring. Moreover, two other hydrophobic residues, Val-22 and Ile-27, are crucial for localization of RIalpha at the neuromuscular junction. These amino acids are involved in the interaction of the Caenorhabditis elegans type Ialpha homologue R(CE) with AKAP(CE) and for in vitro binding of RIalpha to dual A-kinase anchoring protein 1. We also show enrichment of dual A-kinase anchoring protein 1 at the neuromuscular junction, suggesting that it could be responsible for RIalpha tethering at this site.
Figures



Similar articles
-
Characterization of structural features that mediate the tethering of Caenorhabditis elegans protein kinase A to a novel A kinase anchor protein. Insights into the anchoring of PKAI isoforms.J Biol Chem. 2000 Feb 11;275(6):4351-62. doi: 10.1074/jbc.275.6.4351. J Biol Chem. 2000. PMID: 10660605
-
A kinase anchoring protein (AKAP) interaction and dimerization of the RIalpha and RIbeta regulatory subunits of protein kinase a in vivo by the yeast two hybrid system.J Mol Biol. 2003 Mar 28;327(3):609-18. doi: 10.1016/s0022-2836(03)00093-7. J Mol Biol. 2003. PMID: 12634056
-
D-AKAP2, a novel protein kinase A anchoring protein with a putative RGS domain.Proc Natl Acad Sci U S A. 1997 Oct 14;94(21):11184-9. doi: 10.1073/pnas.94.21.11184. Proc Natl Acad Sci U S A. 1997. PMID: 9326583 Free PMC article.
-
Dimerization/docking domain of the type Ialpha regulatory subunit of cAMP-dependent protein kinase. Requirements for dimerization and docking are distinct but overlapping.J Biol Chem. 1998 Dec 25;273(52):35048-55. doi: 10.1074/jbc.273.52.35048. J Biol Chem. 1998. PMID: 9857038
-
Chemoprevention with protein kinase A RIalpha antisense in DMBA-mammary carcinogenesis.Ann N Y Acad Sci. 2005 Nov;1058:255-64. doi: 10.1196/annals.1359.038. Ann N Y Acad Sci. 2005. PMID: 16394142 Review.
Cited by
-
USF2 inhibits C/EBP-mediated transcriptional regulation of the RIIbeta subunit of cAMP-dependent protein kinase.BMC Mol Biol. 2002 Jun 21;3:10. doi: 10.1186/1471-2199-3-10. Epub 2002 Jun 21. BMC Mol Biol. 2002. PMID: 12086590 Free PMC article.
-
USF1 promotes the development of knee osteoarthritis by activating the NF-κB signaling pathway.Exp Ther Med. 2018 Oct;16(4):3518-3524. doi: 10.3892/etm.2018.6608. Epub 2018 Aug 14. Exp Ther Med. 2018. PMID: 30233704 Free PMC article.
-
PKA, PKC, and AKAP localization in and around the neuromuscular junction.BMC Neurosci. 2001;2:17. doi: 10.1186/1471-2202-2-17. Epub 2001 Oct 23. BMC Neurosci. 2001. PMID: 11716788 Free PMC article.
-
Data recovery and integration from public databases uncovers transformation-specific transcriptional downregulation of cAMP-PKA pathway-encoding genes.BMC Bioinformatics. 2009 Oct 15;10 Suppl 12(Suppl 12):S1. doi: 10.1186/1471-2105-10-S12-S1. BMC Bioinformatics. 2009. PMID: 19828069 Free PMC article.
-
Designing isoform-specific peptide disruptors of protein kinase A localization.Proc Natl Acad Sci U S A. 2003 Apr 1;100(7):4072-7. doi: 10.1073/pnas.2628038100. Epub 2003 Mar 19. Proc Natl Acad Sci U S A. 2003. PMID: 12646696 Free PMC article.
References
-
- Colledge M, Scott J D. Trends Cell Biol. 1999;9:216–221. - PubMed
-
- Dell'Acqua M L, Scott J D. J Biol Chem. 1997;272:12881–12884. - PubMed
-
- Rubin C S, Erlichman J, Rosen O M. J Biol Chem. 1972;247:6135–6139. - PubMed
-
- Skålhegg B S, Taskén K, Hansson V, Huitfeldt H S, Jahnsen T, Lea T. Science. 1994;263:84–87. - PubMed
-
- Miki K, Eddy E M. J Biol Chem. 1998;273:34384–34390. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

