In vivo restoration of laminin 5 beta 3 expression and function in junctional epidermolysis bullosa
- PMID: 11296269
- PMCID: PMC33186
- DOI: 10.1073/pnas.091484998
In vivo restoration of laminin 5 beta 3 expression and function in junctional epidermolysis bullosa
Abstract
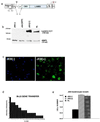
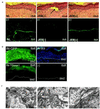
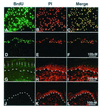
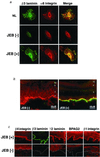
The blistering disorder, lethal junctional epidermolysis bullosa (JEB), can result from mutations in the LAMB3 gene, which encodes laminin 5 beta3 (beta3). Appropriate expression of LAMbeta3 in JEB skin tissue could potentially ameliorate the symptoms of the underlying disease. To explore the utility of this therapeutic approach, primary keratinocytes from six unrelated JEB patients were transduced with a retroviral vector encoding beta3 and used to regenerate human skin on severe combined immunodeficient (SCID) mice. Tissue regenerated from beta3-transduced JEB keratinocytes produced phenotypically normal skin characterized by sustained beta3 expression and the formation of hemidesmosomes. Additionally, beta3 gene transfer corrected the distribution of a number of important basement membrane zone proteins including BPAG2, integrins beta4/beta1, and laminins alpha3/gamma2. Skin produced from beta3-negative (beta3[-]) JEB cells mimicked the hallmarks of the disease state and did not exhibit any of the aforementioned traits. Therefore, by effecting therapeutic gene transfer to beta3-deficient primary keratinocytes, it is possible to produce healthy, normal skin tissue in vivo. These data support the utility of gene therapy for JEB and highlight the potential for gene delivery in the treatment of human genetic skin disease.
Figures
References
-
- Uitto J, Eady R, Fine J D, Feder M, Dart J. J Invest Dermatol. 2000;114:734–737. - PubMed
-
- Akiyama M. Int J Dermatol. 1998;37:722–728. - PubMed
-
- Bale S J, Doyle S Z. J Invest Dermatol. 1994;102:49S–50S. - PubMed
-
- Uitto J, Pulkkinen L, McLean W H. Mol Med Today. 1997;3:457–465. - PubMed
-
- Marinkovich M P. Dermatol Clin. 1999;17:473–485. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

