The generation, migration, and differentiation of olfactory neurons in the adult primate brain
- PMID: 11296302
- PMCID: PMC31906
- DOI: 10.1073/pnas.081074998
The generation, migration, and differentiation of olfactory neurons in the adult primate brain
Abstract
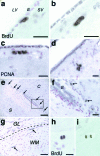
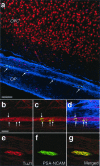
In adult rodents, neural progenitor cells in the subependymal (SZ) zone of the lateral cerebral ventricle generate neuroblasts that migrate in chains via the rostral migratory stream (RMS) into the olfactory bulb (OB), where they differentiate into interneurons. However, the existence of this neurogenic migratory system in other mammals has remained unknown. Here, we report the presence of a homologue of the rodent SZ/RMS in the adult macaque monkey, a nonhuman Old World primate with a relatively smaller OB. Our results-obtained by using combined immunohistochemical detection of a marker for DNA replication (5-bromodeoxyuridine) and several cell type-specific markers-indicate that dividing cells in the adult monkey SZ generate neuroblasts that undergo restricted chain migration over an extended distance of more than 2 cm to the OB and differentiate into granule interneurons. These findings in a nonhuman primate extend and support the use of the SZ/RMS as a model system for studying neural regenerative mechanisms in the human brain.
Figures


References
-
- Jacobson M. Developmental Neurobiology. New York: Plenum; 1991.
-
- Kaplan M S, Hinds J W. Science. 1977;197:1092–1094. - PubMed
-
- Bayer S A, Yackel J W, Puri P S. Science. 1982;216:890–892. - PubMed
-
- Stanfield B B, Trice J E. Exp Brain Res. 1988;72:399–406. - PubMed
-
- Altman J. J Comp Neurol. 1969;137:433–458. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

