Human single-chain Fv intrabodies counteract in situ huntingtin aggregation in cellular models of Huntington's disease
- PMID: 11296304
- PMCID: PMC31908
- DOI: 10.1073/pnas.071058398
Human single-chain Fv intrabodies counteract in situ huntingtin aggregation in cellular models of Huntington's disease
Abstract
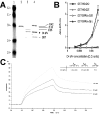
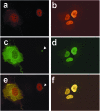
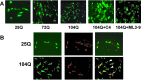
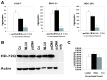
This investigation was pursued to test the use of intracellular antibodies (intrabodies) as a means of blocking the pathogenesis of Huntington's disease (HD). HD is characterized by abnormally elongated polyglutamine near the N terminus of the huntingtin protein, which induces pathological protein-protein interactions and aggregate formation by huntingtin or its exon 1-containing fragments. Selection from a large human phage display library yielded a single-chain Fv (sFv) antibody specific for the 17 N-terminal residues of huntingtin, adjacent to the polyglutamine in HD exon 1. This anti-huntingtin sFv intrabody was tested in a cellular model of the disease in which huntingtin exon 1 had been fused to green fluorescent protein (GFP). Expression of expanded repeat HD-polyQ-GFP in transfected cells shows perinuclear aggregation similar to human HD pathology, which worsens with increasing polyglutamine length; the number of aggregates in these transfected cells provided a quantifiable model of HD for this study. Coexpression of anti-huntingtin sFv intrabodies with the abnormal huntingtin-GFP fusion protein dramatically reduced the number of aggregates, compared with controls lacking the intrabody. Anti-huntingtin sFv fused with a nuclear localization signal retargeted huntingtin analogues to cell nuclei, providing further evidence of the anti-huntingtin sFv specificity and of its capacity to redirect the subcellular localization of exon 1. This study suggests that intrabody-mediated modulation of abnormal neuronal proteins may contribute to the treatment of neurodegenerative diseases such as HD, Alzheimer's, Parkinson's, prion disease, and the spinocerebellar ataxias.
Figures
Similar articles
-
Suppression of Huntington's disease pathology in Drosophila by human single-chain Fv antibodies.Proc Natl Acad Sci U S A. 2005 Aug 9;102(32):11563-8. doi: 10.1073/pnas.0505321102. Epub 2005 Aug 1. Proc Natl Acad Sci U S A. 2005. PMID: 16061794 Free PMC article.
-
A single-chain Fv intrabody provides functional protection against the effects of mutant protein in an organotypic slice culture model of Huntington's disease.Brain Res Mol Brain Res. 2004 Feb 5;121(1-2):141-5. doi: 10.1016/j.molbrainres.2003.11.011. Brain Res Mol Brain Res. 2004. PMID: 14969746
-
Potent inhibition of huntingtin aggregation and cytotoxicity by a disulfide bond-free single-domain intracellular antibody.Proc Natl Acad Sci U S A. 2004 Dec 21;101(51):17616-21. doi: 10.1073/pnas.0408134101. Epub 2004 Dec 14. Proc Natl Acad Sci U S A. 2004. PMID: 15598740 Free PMC article.
-
The selective vulnerability of nerve cells in Huntington's disease.Neuropathol Appl Neurobiol. 2001 Feb;27(1):1-21. doi: 10.1046/j.0305-1846.2001.00299.x. Neuropathol Appl Neurobiol. 2001. PMID: 11298997 Review.
-
Huntingtin-protein interactions and the pathogenesis of Huntington's disease.Trends Genet. 2004 Mar;20(3):146-54. doi: 10.1016/j.tig.2004.01.008. Trends Genet. 2004. PMID: 15036808 Review.
Cited by
-
Effects of intracellular expression of anti-huntingtin antibodies of various specificities on mutant huntingtin aggregation and toxicity.Proc Natl Acad Sci U S A. 2002 Jan 22;99(2):1002-7. doi: 10.1073/pnas.022631799. Epub 2002 Jan 15. Proc Natl Acad Sci U S A. 2002. PMID: 11792860 Free PMC article.
-
Intrabody targeting vascular endothelial growth factor receptor-2 mediates downregulation of surface localization.Cancer Gene Ther. 2017 Jan;24(1):33-37. doi: 10.1038/cgt.2016.76. Epub 2016 Dec 16. Cancer Gene Ther. 2017. PMID: 27982020
-
Protein Misfolding and Aggregation as a Therapeutic Target for Polyglutamine Diseases.Brain Sci. 2017 Oct 11;7(10):128. doi: 10.3390/brainsci7100128. Brain Sci. 2017. PMID: 29019918 Free PMC article. Review.
-
The emerging role of the first 17 amino acids of huntingtin in Huntington's disease.Biomol Concepts. 2015 Mar;6(1):33-46. doi: 10.1515/bmc-2015-0001. Biomol Concepts. 2015. PMID: 25741791 Free PMC article. Review.
-
Differential nuclear localization of complexes may underlie in vivo intrabody efficacy in Huntington's disease.Protein Eng Des Sel. 2014 Oct;27(10):359-63. doi: 10.1093/protein/gzu041. Protein Eng Des Sel. 2014. PMID: 25301961 Free PMC article.
References
-
- The Huntington's Disease Collaborative Research Group. Cell. 1993;72:971–983. - PubMed
-
- Duyao M P, Auerbach A B, Ryan A, Persichetti F, Barnes G T, McNeil S M, Ge P, Vonsattel J P, Gusella J F, Joyner A L, et al. Science. 1995;269:407–410. - PubMed
-
- Nasir J, Floresco S B, O'Kusky J R, Diewert V M, Richman J M, Zeisler J, Borowski A, Marth J D, Phillips A G, Hayden M R. Cell. 1995;81:811–823. - PubMed
-
- Zeitlin S, Liu J P, Chapman D L, Papaioannou V E, Efstratiadis A. Nat Genet. 1995;11:155–163. - PubMed
-
- Mangiarini L, Sathasivam K, Seller M, Cozens B, Harper A, Hetherington C, Lawton M, Trottier Y, Lehrach H, Davies S W, et al. Cell. 1996;87:493–506. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

