NMDA-evoked calcium transients and currents in the suprachiasmatic nucleus: gating by the circadian system
- PMID: 11298803
- PMCID: PMC2577309
- DOI: 10.1046/j.0953-816x.2001.01517.x
NMDA-evoked calcium transients and currents in the suprachiasmatic nucleus: gating by the circadian system
Abstract
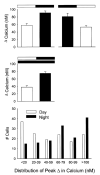
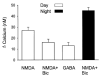
A variety of evidence suggests that the effects of light on the mammalian circadian system are mediated by glutamatergic mechanisms and that the N-methyl- D-aspartate (NMDA) receptor plays an important role in this regulation. One of the fundamental features of circadian oscillators is that their response to environmental stimulation varies depending on the phase of the daily cycle when the stimuli are applied. For example, the same light treatment, which can produce phase shifts of the oscillator when applied during subjective night, has no effect when applied during the subjective day in animals held in constant darkness (DD). We examined the hypothesis that the effects of NMDA on neurons in the suprachiasmatic nucleus (SCN) also vary from day to night. Optical techniques were utilized to estimate NMDA-induced calcium (Ca2+) changes in SCN cells. The resulting data indicate that there was a daily rhythm in the magnitude and duration of NMDA-induced Ca2+ transients. The phase of this rhythm was determined by the light-dark cycle to which the rats were exposed with the Ca2+ transients peaking during the night. This rhythm continued when animals were held in DD. gamma-Aminobutyric acid (GABA)ergic mechanisms modulated the NMDA response but were not responsible for the rhythm. Finally, there was a rhythm in NMDA-evoked currents in SCN neurons that also peaked during the night. This study provides the first evidence for a circadian oscillation in NMDA-evoked Ca2+ transients in SCN cells. This rhythm may play an important role in determining the periodic sensitivity of the circadian systems response to light.
Figures



References
-
- Alberi S, Dauphin MD, Dreifuss JJ, Raggenbass M. Whole-cell NMDA-evoked current in suprachiasmatic neurones of the rat: modulation by extracellular calcium ions. Brain Res. 1997;745:55–66. - PubMed
-
- Block GD, Khalsa SB, McMahon DG, Michel S, Guesz M. Biological clocks in the retina: cellular mechanisms of biological timekeeping. Int. Rev. Cytology. 1993;146:83–144. - PubMed
-
- Burgard EC, Hablitz JJ. Developmental changes in the voltage-dependence of neocortical NMDA responses. Dev. Brain Res. 1994;80:275–278. - PubMed
-
- Cagampang FR, Rattray M, Campbell IC, Powell JF, Coen CW. Variation in the expression of the mRNA for protein kinase C isoforms in the rat suprachiasmatic nuclei, caudate putamen and cerebral cortex. Mol. Brain Res. 1998;53:277–284. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

