Developmental and stress regulation of RCI2A and RCI2B, two cold-inducible genes of arabidopsis encoding highly conserved hydrophobic proteins
- PMID: 11299347
- PMCID: PMC88823
- DOI: 10.1104/pp.125.4.1655
Developmental and stress regulation of RCI2A and RCI2B, two cold-inducible genes of arabidopsis encoding highly conserved hydrophobic proteins
Abstract
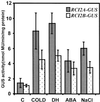
The capability of most higher plants to tolerate environmental conditions strongly depends on their developmental stage. In addition, environmental factors have pleiotropic effects on many developmental processes. The interaction between plant development and environmental conditions implies that some genes must be regulated by both environmental factors and developmental cues. To understand their developmental regulation and obtain possible clues on their functions, we have isolated genomic clones for RCI2A and RCI2B, two genes from Arabidopsis ecotype Columbia (Col), whose expression is induced in response to low temperature, dehydration, salt stress, and abscisic acid. The promoters of RCI2A and RCI2B were fused to the uidA (GUS)-coding sequence and the resulting constructs used to transform Arabidopsis. GUS activity was analyzed in transgenic plants during development under both stressed and unstressed conditions. Transgenic plants with either the RCI2A or RCI2B promoter showed strong GUS expression during the first stages of seed development and germination, in vascular bundles, pollen, and most interestingly in guard cells. When transgenic plants were exposed to low temperature, dehydration, salt stress, or abscisic acid, reporter gene expression was induced in most tissues. These results indicate that RCI2A and RCI2B are regulated at transcriptional level during plant development and in response to different environmental stimuli and treatments. The potential role of RCI2A and RCI2B in plant development and stress response is discussed.
Figures




References
-
- Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K. Current Protocols in Molecular Biology, Suppl 20. New York: John Wiley & Sons; 1992.
-
- Baker SS, Wilhelm KS, Thomashow MF. The 5′-region of Arabidopsis thaliana cor15a has cis-acting elements that confer cold-, drought- and ABA-regulated gene expression. Plant Mol Biol. 1994;24:701–713. - PubMed
-
- Bechtold N, Ellis J, Pelletier G. In planta Agrobacterium mediated gene transfer by infiltration of adult Arabidopsis thaliana plants. CR Acad Sci Paris, Sci la Vie/Life Sci. 1993;316:1194–1199.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

