Stimulation of protein kinase C-dependent and -independent signaling pathways by bistratene A in intestinal epithelial cells
- PMID: 11301042
- PMCID: PMC3601670
- DOI: 10.1016/s0006-2952(01)00596-2
Stimulation of protein kinase C-dependent and -independent signaling pathways by bistratene A in intestinal epithelial cells
Abstract
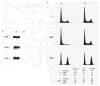
The marine toxin bistratene A (BisA) potently induces cytostasis and differentiation in a variety of systems. Evidence that BisA is a selective activator of protein kinase C (PKC) delta implicates PKC delta signaling in the negative growth-regulatory effects of this agent. The current study further investigates the signaling pathways activated by BisA by comparing its effects with those of the PKC agonist phorbol 12-myristate 13-acetate (PMA) in the IEC-18 intestinal crypt cell line. Both BisA and PMA induced cell cycle arrest in these cells, albeit with different kinetics. While BisA produced sustained cell cycle arrest in G(0)/G(1) and G(2)/M, the effects of PMA were transient and involved mainly a G(0)/G(1) blockade. BisA also produced apoptosis in a proportion of the population, an effect not seen with PMA. Both agents induced membrane translocation/activation of PKC, with BisA translocating only PKC delta and PMA translocating PKC alpha, delta, and epsilon in these cells. Notably, while depletion of PKC alpha, delta, and epsilon abrogated the cell cycle-specific effects of PMA in IEC-18 cells, the absence of these PKC isozymes failed to inhibit BisA-induced G(0)/G(1) and G(2)/M arrest or apoptosis. The cell cycle inhibitory and apoptotic effects of BisA, therefore, appear to be PKC-independent in IEC-18 cells. On the other hand, BisA and PMA both promoted PKC-dependent activation of Erk 1 and 2 in this system. Thus, intestinal epithelial cells respond to BisA through activation of at least two signaling pathways: a PKC delta-dependent pathway, which leads to activation of mitogen-activated protein kinase and possibly cytostasis in the appropriate context, and a PKC-independent pathway, which induces both cell cycle arrest in G(0)/G(1) and G(2)/M and apoptosis through as yet unknown mechanisms.
Figures
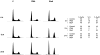
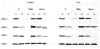


References
-
- Stanwell C, Gescher A, Watters D. Cytostatic and cytotoxic properties of the marine product bistratene A and analysis of the role of protein kinase C in its mode of action. Biochem Pharmacol. 1993;45:1753–61. - PubMed
-
- Griffiths G, Garrone B, Deacon E, Owen P, Pongracz J, Mead G, Bradwell A, Watters D, Lord J. The polyether bistratene A activates protein kinase C-δ and induces growth arrest in HL60 cells. Biochem Biophys Res Commun. 1996;222:802–8. - PubMed
-
- Watters D, Garrone B, Coomer J, Johnson WE, Brown G, Parsons P. Stimulation of melanogenesis in a human melanoma cell line by bistratene A. Biochem Pharmacol. 1998;55:1691–9. - PubMed
-
- Watters D, Marshall K, Hamilton S, Michael J, McArthur M, Seymour G, Hawkins C, Gardiner R, Lavin M. The bistratenes: new cytotoxic marine macrolides which induce some properties indicative of differentiation in HL-60 cells. Biochem Pharmacol. 1990;39:1609–14. - PubMed
-
- Watters D, Garrone B, Gobert G, Williams S, Gardiner R, Lavin M. Bistratene A causes phosphorylation of talin and redistribution of actin microfilaments in fibroblasts: possible role for PKC-δ. Exp Cell Res. 1996;229:327–35. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

