Short-term measures of relative efficacy predict longer-term reductions in human immunodeficiency virus type 1 RNA levels following nelfinavir monotherapy
- PMID: 11302807
- PMCID: PMC90485
- DOI: 10.1128/AAC.45.5.1438-1443.2001
Short-term measures of relative efficacy predict longer-term reductions in human immunodeficiency virus type 1 RNA levels following nelfinavir monotherapy
Abstract
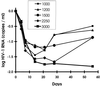
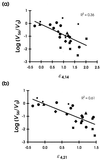
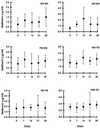
We calculated the relative efficacy of treatment, defined as the rate of decline of virus levels in plasma during treatment relative to the rate of decline during highly potent combination therapy, in human immunodeficiency virus type 1 (HIV-1) patients treated for 56 days with different doses of the protease inhibitor nelfinavir. Relative efficacies based on the rate of decline of HIV-1 RNA levels in plasma over the first 14 to 21 days correlated with drug dose and viral load reduction by day 56. Calculation of relative treatment efficacies over the first 2 to 3 weeks of treatment can allow rapid assessment of new antiretroviral agents and dosing regimens, reducing the need to keep subjects in clinical trials on monotherapy for prolonged periods of time. Relative efficacy may also serve as a measure of treatment efficacy in patients in initiating established therapies.
Figures
Similar articles
-
Virological efficacy and plasma drug concentrations of nelfinavir plus saquinavir as salvage therapy in HIV-infected patients refractory to standard triple therapy.Eur J Med Res. 1999 Feb 25;4(2):54-8. Eur J Med Res. 1999. PMID: 10066640 Clinical Trial.
-
Diverse pattern of protease inhibitor resistance mutations in HIV-1 infected patients failing nelfinavir.J Med Virol. 2005 Aug;76(4):447-51. doi: 10.1002/jmv.20381. J Med Virol. 2005. PMID: 15977242
-
Nelfinavir, efavirenz, or both after the failure of nucleoside treatment of HIV infection.N Engl J Med. 2001 Aug 9;345(6):398-407. doi: 10.1056/NEJM200108093450602. N Engl J Med. 2001. PMID: 11496850 Clinical Trial.
-
Phenotypic hypersusceptibility to multiple protease inhibitors and low replicative capacity in patients who are chronically infected with human immunodeficiency virus type 1.J Virol. 2005 May;79(10):5907-13. doi: 10.1128/JVI.79.10.5907-5913.2005. J Virol. 2005. PMID: 15857976 Free PMC article.
-
Measuring viral load in the clinical setting.J Acquir Immune Defic Syndr Hum Retrovirol. 1995;10 Suppl 1:S34-40. J Acquir Immune Defic Syndr Hum Retrovirol. 1995. PMID: 8595506 Review.
Cited by
-
Predicting virological decay in patients starting combination antiretroviral therapy.AIDS. 2016 Jul 17;30(11):1817-27. doi: 10.1097/QAD.0000000000001125. AIDS. 2016. PMID: 27124894 Free PMC article.
-
Viral load Reduction in SHIV-Positive Nonhuman Primates via Long-Acting Subcutaneous Tenofovir Alafenamide Fumarate Release from a Nanofluidic Implant.Pharmaceutics. 2020 Oct 17;12(10):981. doi: 10.3390/pharmaceutics12100981. Pharmaceutics. 2020. PMID: 33080776 Free PMC article.
-
Pharmacokinetics, pharmacodynamics and drug interaction potential of enfuvirtide.Clin Pharmacokinet. 2005;44(2):175-86. doi: 10.2165/00003088-200544020-00003. Clin Pharmacokinet. 2005. PMID: 15656696 Review.
References
-
- Angel J B, Kumar A, Parato K, Filion L G, Diaz-Mitoma F, Daftarian P, Pham B, Sun E, Leonard J M, Cameron D W. Improvement in cell-mediated immune function during potent anti-human immunodeficiency virus therapy with ritonavir plus saquinavir. J Infect Dis. 1998;177:898–904. - PubMed
-
- Cao Y, Ho D D, Todd J, Kokka R, Urdea M, Lifson J D, Piatak M, Jr, Chen S, Hahn B H, Saag M S. Clinical evaluation of branched DNA signal amplification for quantifying HIV type 1 in human plasma. AIDS Res Hum Retrovir. 1995;11:353–361. - PubMed
-
- Chiesi A, Mocroft A, Dally L G, Miller V, Katlama C, Ledergerber B, Pedersen C, Phillips A N, Arcieri R, Lundgren J D. Regional survival differences across Europe in HIV-positive people: the EuroSIDA study. AIDS. 1999;13:2281–2288. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

