Helicobacter pylori infection induced alteration of gene expression in human gastric cells
- PMID: 11302954
- PMCID: PMC1728271
- DOI: 10.1136/gut.48.5.598
Helicobacter pylori infection induced alteration of gene expression in human gastric cells
Abstract
Background: Helicobacter pylori, a human pathogen responsible for many digestive disorders, induces complex changes in patterns of gene expression in infected tissues. cDNA expression arrays provide a useful tool for studying these complex phenomena.
Aim: To identify genes that showed altered expression after H pylori infection of human gastric cells compared with uninfected controls.
Methods: The gastric adenocarcinoma cell line AGS was cocultivated with H pylori. Growth of infected cells was determined by trypan blue exclusion assay. Complementary DNA probes derived from H pylori treated and untreated cells were hybridised to two identical Atlas human cDNA expression arrays, and those genes with altered expression levels were identified. A real time quantitative reverse transcription-polymerase chain reaction assay was used to better define expression patterns of these genes in endoscopically gastric mucosal biopsies with and without H pylori infection.
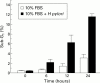
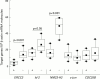
Results: Over 24 hours, coincubation with H pylori inhibited AGS cell growth but did not cause a noticeable degree of cell death. H pylori treatment altered the pattern of gene expression in AGS cells. We identified 21 overexpressed genes and 17 suppressed genes from the cDNA expression arrays. The majority of genes were transcription factors such as c-jun, BTEB2, and ETR101. Other genes were involved in signal transduction pathways, such as MAP kinase, interleukin 5, and insulin-like growth factor. Genes involved in cell cycle regulation and differentiation, such as CDC25B and NM23-H2, were also identified. In patients with H pylori infection (n=20), there was a significant difference for ERCC3, Id-2, and NM23-H2 mRNA levels in infected gastric mucosa compared with uninfected gastric mucosa in patients without peptic diseases (n=20) (ERCC3 4.75 molecules/10(4) beta-actin mRNA molecules v 13.65, p<0.001; Id-2 16.1 v 23.4, p<0.05; NM23-H2 17.5 v 45.5, p<0.001). There was no significant difference between mRNA levels of c-jun and CDC25B in H pylori colonised gastric mucosa and uninfected mucosa.
Conclusion: We demonstrated that H pylori infection caused alteration of gene expression in AGS cells. The differential hybridisation technique of Atlas human cDNA expression array is a useful method to identify host genes involved in pathogenic mechanisms in H pylori infection.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
